Abstract
Dehydroepiandrosterone sulfate (DHEAS) is prenatally secreted by the fetal adrenal, is an indicator of adrenarche from late childhood onward and is a marker of the individual hormonal milieu in the adult. The regulation of DHEAS secretion is still poorly understood. We postulated that serum DHEAS concentrations in children may be related to fetal growth. To test this hypothesis, serum DHEAS was measured at a median age of 8.2 y (range 5.8-16.0 y) in 13 pairs of discordant siblings after twin (n = 8), triplet(n = 4), or quadruplet (n = 1) pregnancy. At birth, one of each pair was small for gestational age (SGA) and the other had an appropriate weight (AGA); weight of the smallest infant was a median 67% (range 33-80%) of that of the largest sibling. In all 10 pairs with similar weight (≤ 1 SD difference) at the time of sampling, serum DHEAS concentration in the SGA child was higher (median 2-fold increase; range 1.1-7; p = 0.002) than in the AGA sibling. Conversely, in the 3 pairs with still discordant weight (>2 SD difference), serum DHEAS levels in SGA children were lower than in AGA children. In conclusion, the presented findings, which account for both prenatal and postnatal weight gain, unmask a link between adrenarche and fetal growth. This relationship further supports the concept of early endocrine “programming” and extends this principle to adrenarche.
Similar content being viewed by others
Main
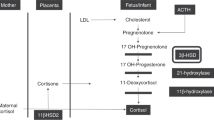
Intrauterine growth retardation is known to be associated with hypoplasia of the fetal adrenal zone and with lower fetal serum concentrations of DHEAS(1, 2). The involution of the fetal adrenal after birth is reflected by a virtual disappearance of DHEAS from the circulation until adrenarche.
Adrenarche occurs exclusively in primate species. This phenomenon correlates histologically with the appearance and growth of the zona reticularis, within the adrenal cortex, and seems to be independent of pubertal development. Endocrine hallmarks of adrenarche are increasing circulating levels of DHEA, DHEAS, and androstenedione. DHEAS has the longer half-life in the circulation (8-11 h) and does not exhibit a circadian rhythm(3–5). DHEAS has been identified as a highly specific marker of the individual hormonal milieu in the adult(7).
The regulation of DHEAS secretion is still poorly understood. ACTH availability appears to be a prerequisite. Other possible regulators of adrenal androgen secretion are prolactin, dopamine, IGF-I, and a hitherto unidentified adrenal-androgen-stimulating hormone of pituitary origin. Nutritional state and intercurrent illness are also known to influence plasma DHEAS levels(3–8).
Recently, Barker et al.(9) reported increased rates of cardiovascular disease and non-insulin-dependent diabetes in adults born SGA and launched the concept of “programming” in fetal life. In this concept, the growth-retarded fetus adapts to undernutrition and survives by altering some endocrine and metabolic set points, which appear to remain altered postnatally(9, 10).
We postulated that fetal growth may be a modulator of adrenarche and tested this hypothesis by examining serum DHEAS concentrations in pairs of children who were born as discordant siblings from the same pregnancy.
METHODS
Thirteen pairs of discordant siblings, who were born in our hospital from twin (n = 8), triplet (n = 4), or quadruplet (n= 1) pregnancies were available for examination (after informed consent of both parents) at a median age of 8.2 y (range 5.8-16.0 y). At birth, one of each pair was SGA with a weight or length ≤ 2 SD(11), and the other was of appropriate weight (AGA). Birth weight of the smallest infant was a median 67% (range 33-80%) of that of the largest sibling.
Clinical examination of the children included measurement of height and weight, and pubertal staging according to Tanner. None of the children presented evidence of a systemic or syndromic disorder.
Serum DHEAS concentrations of SGA children and their AGA siblings were measured. In triplets and quadruplets, serum DHEAS of the siblings with the lowest and highest birth weight were compared. Serum levels of DHEAS were measured by RIA using the DSL DHEAS Radioimmunoassay Kit (Diagnostic Systems Laboratories, Webster, TX).
The intraassay coefficient of variation was 5.1% at 0.6 mmol/L and 4.1% at 6.1 mmol/L. The detection limit was 0.05 mmol/L. Reference ranges of DHEAS during childhood and adolescence were obtained from Reiter et al.(12). Paired t test was used for statistical comparison.
RESULTS
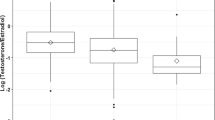
Clinical data and serum DHEAS concentrations are summarized in Table 1 and Figure 1. In all 10 pairs with similar weight (≤1 SD difference) at the time of sampling, serum DHEAS concentrations in SGA children were higher (median 2-fold increase; range 1.1-7; p = 0.002) than in AGA siblings. Conversely, in the 3 pairs with still discordant weight (>2 SD difference), serum DHEAS levels in SGA children were lower than in AGA children.
DISCUSSION
In 10 pairs of children, born as discordant siblings but matched for current weight, serum DHEAS was consistently higher in the SGA child, compared with the AGA sibling. In three SGA children who were still discordant in weight, serum DHEAS was lower than in their AGA sibling.
During late childhood and adolescence, serum DHEAS is positively related to age and current weight(4, 5, 8). The study design and the adjustment for current weight unmasked the association between size at birth and serum DHEAS in childhood: prenatal growth restriction appears to result in a more pronounced adrenarche. It will be of interest to differentiate whether the relative increase of serum DHEAS results from an acceleration or from an amplification of adrenarche, as the former may be a temporary phenomenon limited to childhood and adolescence, whereas the latter may have lifelong consequences(13).
In siblings with still differing weight, the modulation of adrenarche by prenatal growth (negative relationship) appears to be overcome by the influence of current weight on circulating DHEAS (positive relationship).
The pathophysiologic mechanism underlying our findings is at present unclear. Both the fetal adrenal and the zona reticularis of the adult adrenal cortex are characterized by a low level of expression of 3β-hydroxysteroid dehydrogenase(14). It is therefore possible that the function of this enzyme plays a crucial role in the association between adrenarche and fetal growth.
Children born SGA present a particular pattern of growth and bone maturation. A subgroup of them is known to have an acceleration of bone maturation in late childhood, independently of pubertal development(15). The data reported here suggest that exaggerated adrenarche may participate in the pathogenesis of this phenomenon.
In conclusion, the presented findings, which account for both prenatal and postnatal weight gain, unmask a link between adrenarche and fetal growth. This relationship further supports the concept of early endocrine“programming” and extends this principle to adrenarche.
Abbreviations
- AGA:
-
appropriate for gestational age
- SGA:
-
small for gestational age
- DHEAS:
-
dehydroepiandrosterone sulfate
- DHEA:
-
dehydroepiandrosterone
References
Naeye RL 1965 Malnutrition: probable cause of fetal growth retardation. Arch Pathol 79: 284–291
Turnispeed MR, Bentley K, Reynolds JW 1976 Serum dehydroepiandrosterone sulfate in premature infants and infants with intrauterine growth retardation. J Clin Endocrinol Metab 43: 1219–1225
Grumbach MM, Styne DM 1992 Williams Textbook of Endocrinology, WB Saunders, Philadelphia, pp 1139–1221
Orth DN, Kovacs WJ, Debold CR 1992 Williams Textbook of Endocrinology, WB Saunders, Philadelphia, pp 489–619
Parker LN 1991 Control of adrenal androgen secretion. Endocrinol Metab Clin North Am 20: 401–421
Van den Berghe G, de Zegher F, Wouters P, Schetz M, Verwaest C, Ferdinande P, Lauwers P 1995 Dehydroepiandrosterone sulphate in critical illness: effect of dopamine. Clin Endocrinol 43: 457–463
Thomas G, Frenoy N, Legrain S, Sebag-Lanoe R, Baulieu EE, Debuire B 1994 Serum dehydroepiandrosterone sulfate levels as an individual marker. J Clin Endocrinol Metab 79: 1273–1276
Treasure J, Wheeler MJ, Safieh B, Russell GF 1985 Anorexia nervosa and the adrenal: the effect of weight gain. J Psychiatr Res 19: 221–225
Barker DJP, Gluckman PD, Godrey KM, Harding JE, Owens JA, Robinson JS 1993 Fetal nutrition and cardiovascular disease in adult life. Lancet 341: 938–41
Barker DJP, Hales CN, Fall CH, Osmond C, Phipps K, Clark PM 1993 Type 2 (non-insulin-dependent) diabetes mellitus, hypertension and hyperlipidaemia (syndrome X): relation to reduced fetal growth. Diabetologia 36: 62–67
Lawrence C, Fryer JG, Karlberg P, Niklasson A, Ericson A 1989 Modelling of reference, values for size at birth. Acta Paediatr Scand Suppl 350: 55–69
Reiter EO, Fuldauer VG, Root AW 1977 Secretion of the adrenal androgen, dehydroepiandrosterone sulfate, during normal infancy, childhood, and adolescence, in sick infants, and in children with endocrinologic abnormalities. J Pediatr 90: 766–770
Baulieu EE 1996 Dehydroepiandrosterone (DHEA): a fountain of youth?. J Clin Endocrinol Metab 81: 3147–3151
Endoh A, Kristiansen SB, Casson PR, Buster JE, Hornsby PJ 1996 The zona reticularis is the site of biosynthesis of DHEA and DHEAS in the adult human adrenal cortex, resulting from its low expression of 3β-hydroxysteroid dehydrogenase. J Clin Endocrinol Metab 81: 3558–3565
Tanner JM, Lejarraga H, Cameron N 1975 The natural history of the Silver-Russell syndrome: a longitudinal study of thirty-nine cases. Pediatr Res 9: 611–623
Acknowledgements
The authors thank the concerned children and parents for their participation in this study, Karin Vanweser for logistic and editorial assistance, Professor D. J. P. Barker and Dr. P. M. Clark(Southampton, UK), and Professor P. D. Gluckman (Auckland, NZ) for manuscript review.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Francois, I., De Zegher, F. Adrenarche and Fetal Growth. Pediatr Res 41, 440–442 (1997). https://doi.org/10.1203/00006450-199703000-00023
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/00006450-199703000-00023