Abstract
Data are scarce regarding mineral bioavailability from human milk in older infants who may also be receiving solid foods (beikost). We measured the absorption of Ca, Zn, and Fe in 14 healthy, nonanemic 5-7-mo-old breast-fed infants whose mothers' milk was extrinsically labeled with stable isotopes(44Ca, 70Zn, and 58Fe) of these minerals. In addition, Ca and Zn stable isotopes (46Ca and 67Zn) were administered i.v., and a second isotope of Fe (57Fe) was given orally without food as a non-meal dose. Subjects were not receiving any artificial infant formula or cow's milk, but most (10/14) were receiving beikost. Ca and Zn absorption was calculated using the urinary excretion of the isotopes during the 24 h after dosing (Ca) or their urinary ratio 72 h after dosing (Zn). Fe absorption was calculated using the red blood cell incorporation at 14 d. Fe absorption averaged 20.7 ± 14.8% from the 58Fe given with human milk(geometric mean, 14.8%) and 17.7 ± 15.1% (geometric mean, 11.0%) from the 57Fe non-meal dose. Ca absorption averaged 61.3 ± 22.7% and Zn absorption (n = 10) averaged 49.5 ± 18.5%. Absorption of Fe (natural logarithm) from the non-meal Fe dose (57Fe) but not from the human milk (58Fe) was significantly negatively correlated to serum ferritin (r = -0.70, p = 0.007 versus r = -0.35,p = 0.24). At the intake levels in this study, total daily Fe, Ca, and Zn intakes from beikost were not significantly correlated to their fractional absorption from breast milk, but Fe intake from beikost was significantly negatively correlated to absorption of Fe from the non-meal dose(r = -0.61, p = 0.021). We conclude that minerals are well absorbed from human milk in older infants after the introduction of beikost to the diet.
Similar content being viewed by others
Main
Current recommendations encourage breast-feeding throughout the first year of life, with solid foods (beikost) introduced between 4 and 6 mo of age. However, the rate of mineral absorption from human milk during this time period and the effects of beikost on mineral absorption from human milk are unanswered questions.
Most mass balance studies in infants have used young infants <4 mo of age whose sole source of nutrition was either human milk or infant formula(1, 2). Mineral balance studies are hard to perform in older infants due to problems with sample collection. Nonetheless, the 4-12-mo age period is an important one for mineral acquisition, and interpolation from data in younger infants may not be appropriate(1). Uncertainty as to the mineral needs of older infants has led, for example, to widely varying levels of minerals in infant formulas designed for older infants.
Recently, a two-tracer stable isotope method for measuring Fe incorporation in infants has been described(3–5). This method uses two orally administered Fe isotopes to compare the fraction of Fe which is incorporated into RBCs from a meal with that from a dose given orally separately from a meal (often referred to as a “reference dose”). This fraction is determined from the enrichment of each isotope in a blood sample obtained 14 d after isotope administration.
Dual-isotope techniques for measurement of Ca and Zn absorption, using urinary excretion of oral and i.v. administered stable isotopes of these minerals, have been described and validated(6–12). The dual-tracer technique used for Ca and Zn differs from that used for Fe in that, in the dual-isotope technique, the relative ratio of the oral versus the i.v. isotope is used to determine fractional absorption. Use of these tracer techniques obviate the need for fecal collections. However, perhaps because of concern regarding the difficulty of administering i.v. isotopes to healthy full-term infants, there are no previous reports of their application to studies or Ca and Zn metabolism involving these infants.
In this study, we used these multiple-tracer techniques to measure Ca, Zn, and Fe absorption in 5-7-mo-old infants, most of whom were receiving beikost in addition to human milk. Our goals were: 1) to evaluate the relationship between mineral intake from beikost and mineral absorption in older infants, 2) to evaluate the relationship between Fe absorption and serum ferritin in older infants, and 3) to provide data regarding mineral absorption from human milk in this population group.
METHODS
Study design. Sixteen infants were enrolled in the study and 14 subjects (nine girls and five boys) age 164-225 d (mean age 188 ± 19 d, weight 7.8 ± 1.2 kg) completed the study (see Table 1). Subjects were recruited from the greater Houston area by public advertising and enrolled without prior evaluation of their mineral status. Informed written consent was obtained from the parents of each subject, and the protocol was approved by the Institutional Review Board of Texas Children's Hospital/Baylor College of Medicine. None of the subjects had a history of anemia or other chronic illness. None of the subjects was receiving any multivitamin or Fe-containing vitamin supplement. None of the subjects was ill at the time of the study.
Twenty-four hours before the study, an aliquot of 250 mL of each infant's mother's milk was prepared for use in the study. 58Fe (180 μg),70 Zn (200 μg), and 44Ca (1.5 mg) were added to the milk, mixed using a magnetic stir-bar for 1 h, and refrigerated overnight.
On the morning of the study, infants and their mothers were admitted for a 24-h stay to the Metabolic Research Unit of the Children's Nutrition Research Center in Houston, TX. Infants were not allowed to eat for at least 2 h before the beginning of the study. Infants were weighed, and their recumbent length was measured.
Three milliliters of blood were drawn from an antecubital vein using a 23-g butterfly needle infusion set for Hb, hematocrit, and serum ferritin (analyzed using RIA by a commercial laboratory). After the blood was withdrawn,46 Ca (10 μg) and 67Zn (500 μg) were given i.v. using the same butterfly infusion set. This was done by infusing the Zn first (over 1 min), flushing the catheter with 1 mL saline, then infusing the Ca over 1 min, and finally flushing the infusion set with an additional 1 mL of saline. At the beginning and end of each infusion, the butterfly needle was tested to ensure it remained within the vein as demonstrated by standard clinical criteria, including the ready return of blood into the butterfly tubing when the syringe was removed from the setup.
All infusions were done by one of the investigators (S.A.A.). Two infants initially enrolled in the study were withdrawn because of the inability to obtain i.v. access for the Ca and Zn infusions. No samples or data were collected from these two infants.
At the beginning of the infusion, urine collection was begun using a 24-h bagged collection system. Infants were bottle-fed the 250 mL of breast milk-isotope mixture during the course of the next three meals and the initial 8-12 h of the study. Beikost-containing meals (and nonlabeled human milk if needed) were offered with each bottle of labeled human milk.
Mothers were instructed to feed their infants per the infant's typical home schedule, and foods were made available for the infant which matched the foods usually consumed at home. After the 250-mL breast milk-isotope mixture had been ingested, mothers either bottle-fed their infants additional breast milk, which they had pumped, or breast-fed the infant. All bottles used were pre- and postweighed, and infants were weighed before and after breast feeding.
Approximately 14 h after the beginning of the study, at least 2 h after the last meal, and after all the breast milk-isotope mixture had been ingested, 1.0 mg of 57Fe (as ferrous sulfate) was administered via a syringe into each infant's mouth. No additional food was allowed for 2 h after the57 Fe was given.
After 24 h, the urine collection was discontinued. The infants and their mothers were discharged from the Metabolic Research Unit, and the mothers were instructed to obtain a bagged urine specimen 72-96 h after the initial study. This was, however, unsuccessful in some cases and thus spot urine samples for Zn absorption were not obtained for four of the 14 study infants. Subjects returned to the Metabolic Research Unit 14 d after the initial study. At that time 1 mL of blood was withdrawn from an antecubital vein for Fe isotope-ratio determination.
Dietary methods. After subject recruitment, the dietitian(J.E.S.) contacted each mother and thoroughly reviewed her infant's usual diet. In this interview, the dietitian determined the types of foods (fruit, vegetable, meat, cereal), their source (level of baby food, or table food), and quantity in the infant's usual diet. Based on these data, a menu of food choices was prepared from which the mother selected foods for the infant, while making an effort to reproduce the infant's typical home diet. To verify the foods eaten during the study period, results of the 24-h weighed dietary record were compared with the food pattern obtained from the prestudy dietary interview.
Isotope preparation and sample analysis. 57Fe (96 atom%) and 58Fe (93 atom%), which were produced in Russia, were purchased as the metal and prepared as ferrous sulfate using highly purified sulfuric acid. Isotopes were prepared using ascorbic acid in a 1:1 molar ratio to maintain the Fe in the reduced (Fe2+) state. The final concentration of the tracer solutions of Fe was 1.2 mg/mL (57Fe) and 0.60 mg/mL(58Fe). All Fe concentrations were measured in triplicate by atomic absorption spectroscopy.
70Zn (74 atom%), 67Zn (90 atom%), 46Ca (5 or 30 atom%), and 44Ca (95 or 98 atom%) (produced either in Russia or at Oak Ridge National Laboratories, Oak Ridge, TN) were prepared for human use as the chloride salt in keeping with described methodology(6–12). All isotopes were tested for sterility and pyrogenicity before human use.
For iron, the blood sample of 0.1-0.5 mL was digested in 2-10 mL of concentrated HNO3 in a titration flask on a hot plate at sub-boiling temperature for 24 h. The sample was then dried and redissolved in 1-2 mL of 6 N HCl to make the sample solution for the ion exchange procedure. A polyethylene column 0.4 cm in diameter and 8 cm in length, with a 4-mL reservoir on the top, was filled with anion exchange resin (AG-1 X]8, 100-200 mesh). The resin in the column was cleaned with 4 mL of ultra-clean 6 N HCl and 4 mL of ultra-pure H2O. The column was then reconditioned in 6 N HCl before the sample solution was loaded. After the sample solution had passed through the column, it was washed with 6 mL of 6 N HCl followed by 0.5 mL of 0.5 N HCl before Fe was extracted from the column by 1 mL of 0.5 N HCl. The solution collected was dried and resuspended in 30-50 μL of 3% HNO3 and loaded onto the filament for mass spectrometric analysis.
For Zn, 5 mL of the spot urine were dried in a beaker, then digested in concentrated HNO3 on a hot plate. The samples were redissolved in 6 N HCl and dried before they were dissolved in 4-5 mL of 0.5 N HCl. A glass column of 1 cm in diameter and 18 cm in length was filled with cation exchange resin (AG 50W X8, 100-200 mesh). The column was washed with 40 mL of 6 N HCl and 20 mL of H2O and reconditioned in 0.5 N HCl before the sample solution was loaded onto the column. After the sample solution had gone through, Zn was collected with 30 mL of a mixed reagent of 60% acetone + 0.5 N HCl (by volume). The Zn collection was then dried and redissolved in 1-2 mL of 6 N HCl as a sample solution for the next anion exchange procedure. The anion exchange column for Zn was the same as for Fe. After the sample solution was loaded and passed through, the column was washed with 6 mL of 6 N HCl, followed by 4 mL of 1 N HCl, before Zn was extracted from the column using 4 mL of 0.0005 N HCl.
Five milliliters of urine from the 24-h pool were used for Ca isotope ratio determination. Urine samples were prepared for mass spectrometric analysis for Ca using an oxalate technique originally described by Yergey and co-workers(11, 12).
All samples were analyzed for isotopic enrichment using a Finnigan MAT 261 magnetic sector thermal ionization mass spectrometer. Each sample was manually heated for 10-15 min to approximately 3 A to obtain a total ion current of 5-8× 10-11 A of 56Fe, 44Ca, or 67Zn on the axial Faraday detector. Three blocks of 10 scans each were made. Our method is similar to the method used for reference analysis and is capable of high precision and accuracy for Fe, Ca, and Zn isotope ratio measurement. We obtained a maximum variability (relative SD) of 0.2% for repeated measurements of Fe, Ca, and Zn isotope ratios from the same sample when corrected for fractionation to the naturally occurring 54Fe/56Fe,42 Ca/43Ca, or 64Zn/66Zn ratios(11–14).
Calculations. RBC Fe incorporation was determined as previously described by evaluating the recovery of the orally administered isotopes in blood obtained 14 d after isotope administration(5). Circulating Fe was calculated using a mean blood volume of 80 mL/kg, the measured Hb concentration, and the concentration of Fe in Hb (3.47 mg/g). Percent absorption was calculated assuming that 90% of the absorbed isotope is incorporated into RBCs(3, 13). This value (90%) may be directly determined by administering an isotope of Fe i.v., which was not performed as part of this study(2, 3, 15).
We used the method of Friel et al.(6, 7) to determine Zn absorption from a spot urine sample collected 72-96 h after the infusion/ingestion of the Zn tracers. This method is more accurate for Zn than the 24-h pooled urine method used for Ca(7). The relative recovery of the tracers in the urine is multiplied by 100 to calculate the percentage of Zn absorption. Total urinary Zn was determined by atomic absorption spectroscopy.
Although the use of a spot urine sample has been validated for Zn studies, this method is not reliable for Ca absorption determination(16). We used the ratio of urinary excretion of the Ca isotopes during the 24 h after isotope administration to calculate fractional absorption. Yergey and others have validated this method previously(10, 12, 16).
In most of the infants (11/14), the 24-h urine was collected as a single pooled sample. The enrichment of an i.v. administered isotope in that pool is related to the distribution volume of the tracer and may represent a measure of the high turnover Ca pool of the subject. We have referred to this as the“i.v. distribution pool mass” (IDPM) and calculated it as(17): Equation

Statistical analysis. Linear regression analysis was used to relate mineral absorption to intake from solids or biochemical parameters. Relative SD was calculated as the SD divided by the mean. All data are mean± SD.
RESULTS
Subjects. The age, sex, weight, and total 24-h intake of human milk are shown for the 14 study subjects in Table 1. Daily milk volume averaged 766 mL/d, slightly lower than the daily average of 875 mL/d Dewey et al.(18) reported for breast-fed 7-mo-old infants.
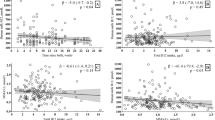
Iron. Results for the Fe absorption studies are shown in Table 2. We found a significant linear relationship between Fe intake from beikost (mg) and (non-meal) 57Fe absorption(natural logarithm); ln(57Feabs) = 0.087·Feintake+ 3.015 (r = -0.67, p = 0.008). The linear relationship between Fe intake from beikost and (human milk-fed) 58Fe absorption(natural logarithm) was not significant; ln(58Feabs) =-0.026·Feintake + 2.88, (r = -0.23, p = 0.42).
There was a close relationship between serum ferritin and the 57Fe absorption (natural logarithm); ln (57Feabs) =-0.012·ferritin + 3.163, r = -0.70, p = 0.007. The relationship between serum ferritin and 58Fe absorption (natural logarithm) was not significant; ln(58Feabs) =-0.05·ferritin + 3.037 (r = -0.35, p = 0.24). Blood was not obtained from one infant for a serum ferritin.
Fe intake from beikost was marginally correlated to serum ferritin,r = 0.43, p = 0.15. If subject 12, who had no Fe intake from beikost and a serum ferritin of 142, is excluded, the relationship is closer, r = 0.55, p = 0.06. Hb and hematocrit were not significantly correlated to 57Fe or 58Fe absorption or their natural logarithms (p > 0.10 for each) or to Fe intake from beikost (p > 0.4 for each).
We calculated Fe absorption (Table 2) using a blood volume estimate of 80 mL/kg. This value is not, however, well established in infants, and was not measured in our study. Values of 65-80 mL/kg are generally used in infants(3, 15, 20). Using a lower value for blood volume will decrease the calculated Fe absorption. For example, if a value of 65 mL/kg was used, 57Fe absorption would be 15.4 ± 14.4% (geometric mean 9.5%) and 58Fe absorption would be 17.5 ± 16.9% (geometric mean 12.8%) in our study subjects.
Zinc and calcium. Results for Zn intake and absorption(n = 10) are shown in Table 3. The mean Zn absorption was 49.5 ± 18.5%. There was no significant correlation between Zn intake from beikost and Zn absorption (r = 0.39,p = 0.26). Urinary Zn excretion averaged 0.14 ± 0.18 mg/d.
Results for Ca absorption are shown in Table 4. The mean absorption was 61.3 ± 22.7%. Urinary Ca excretion averaged 23.4± 17.2 mg/d. Three of the 14 infants had urinary Ca excretions in excess of 4 mg/kg/d. There was no significant correlation between Ca intake from beikost and Ca absorption (r < 0.1). Fractional absorption of Ca was not significantly correlated to fractional absorption of Zn,57 Fe, or 58Fe (r < 0.4 for each).
The IDPM for Ca was determined in 11/14 study subjects and averaged 170± 32 mg/kg. This is approximately that of the IDPM in premature infants(361 ± 69 mg/kg), but about 50% greater than the IDPM in 5-11-y-old children (114 ± 25 mg/kg)(17).
DISCUSSION
Iron. We found that Fe absorption remained high from a dose of58 Fe given with breast milk even in those infants who were receiving beikost. Although the reliability of using a single day's intake to assess dietary Fe intake is limited, the close correlation between 57Fe(non-meal dose) absorption (p = 0.007) but not 58Fe absorption (p = 0.23) and Fe intake from beikost suggests that our Fe intakes approximated usual intakes. We found a significant negative correlation between serum ferritin and 57Fe absorption but not58 Fe absorption. This difference indicates that Fe absorption from a non-meal dose is more closely related to Fe status and Fe intake in 5-7-mo-old infants than Fe absorption from breast milk. The reason for this difference cannot be determined from this study. However, the difference may suggest that the absorption of Fe from human milk is related to specific components of human milk rather than Fe status. Possible reasons for the enhanced Fe absorption from human milk relative to other Fe sources have been reviewed(19).
These results suggest that Fe absorption from supplements given to older infants separately from a meal may have bioavailability comparable to that of human milk, especially in infants with less beikost in their diet or lower serum ferritin levels. However, although the 57Fe given in our study was similar in form (ferrous sulfate) to routine iron supplements, the total dose given was only 1.0 mg, which is well below the dose usually given in supplements. Evaluation of higher dose levels would be needed to determine the absorption of Fe from supplements in 5-7-mo-old infants.
The values for Fe absorption in this study are similar to those in a recent report by Davidsson et al.(21), which also used stable isotopes. Using a blood volume estimate of 65 mL/kg, they reported a geometric mean Fe absorption of 11.8% in eight human milk-fed infants, ages 2-10 mo (mean age 5 mo). This value is virtually identical to the 12.8% geometric mean Fe absorption in our study (using a blood volume of 65 mL/kg). Some of the infants in Davidsson's study had received beikost at home before study enrollment. However, in Davidsson's study, unlike ours, no beikost was provided to the infants on the study day.
The percentage of Fe absorbed from human milk by older infants in this study is substantially less than the value of approximately 50% previously reported for Fe absorption from human milk(19, 22, 23). This difference may be due to the age of the subjects, as greater Fe absorption from human milk may occur during the first months of life than subsequently. Differences between studies in the technique used to determine Fe absorption may also be responsible for the difference in results. High values for Fe absorption were generally obtained indirectly or using nonequilibrated doses of an Fe isotope. Methodologic issues regarding studies of Fe absorption in breast-fed infants have been discussed in detail elsewhere(21, 24).
Levels of other nutrients, such as Ca, can affect Fe absorption(25, 26). In the present study, however, Ca intake from beikost was relatively low and not correlated with Fe absorption. It is unlikely, therefore, that the intake of Ca from beikost was an important factor in Fe absorption in the study infants. It is of note that Fe absorption from both human milk and the non-meal dose in this study was greater than the absorption in 12-mo-old infants from cow's milk(5).
Although our study, and that of Davidsson et al.(21), used doses of 58Fe, which may have been comparable to the total Fe native to the human milk during the study period, our added dose of 180 μg of 58Fe (approximately 190 μg total Fe) was distributed over several feedings, including those which generally contained much larger amounts of Fe from beikost. This may have lowered the absorption of Fe we obtained. In theory, as we achieved more than adequate enrichment of the RBCs with our dosing (mean 4% enrichment), it is likely that a dose of 58Fe as low as 50-100 μg could be used for studies in 2-6-mo-old infants. If given over a 24-h period, the total added Fe would be less than the native human milk Fe intake during this period.
It is also possible that 2 h was an inadequate time period for gastric emptying and that some minerals may have remained in the stomach at the time the non-meal dose of 57Fe was given. However, it is unlikely this effect was large, and 4 h is the maximum practical time period for keeping many small infants without food during waking hours.
Zinc. This study is the first to use a dual-tracer technique to determine fractional Zn absorption in full-term infants. Both absorption and endogenous fecal Zn excretion (not addressed in this study) are important determinants of Zn regulation(26–31). Zinc absorption averaged approximately 50% in the 10 infants studied. We did not find any relationship between Zn intake from beikost and Zn absorption. Although interactions among Zn, Ca, and Fe absorption are well described(26, 28), we did not find any evidence of a relationship between Zn absorption and Ca absorption or Zn absorption and Fe absorption in our study population. Possible reasons for this lack of a relationship are the low levels of these minerals in human milk and the relatively high rates of mineral absorption from human milk. In addition, the range of Zn and Ca intake in this study was relatively small. Studies involving higher levels of Ca and Zn supplementation from beikost would be needed to address the interactive effects more completely.
There are few previous studies of dietary Zn absorption in full-term infants(28, 29). Using a single oral tracer fed to infants receiving a low Zn-containing formula, Ziegler et al.(29) found that fractional absorption averaged 41.1± 7.8% of intake. Using a similar method, Krebs et al.(30) have found (preliminary results) approximately 50% absorption of zinc in 2-4-mo-old human milk-fed infants. Using standard balance techniques, Sievers et al.(31) found net retentions of approximately 100 μg/kg in 3- and 4-mo-old breast-fed term infants with a median retention of 27% of intake.
Calcium. Although we and others have widely used the dual-tracer stable isotope technique to study Ca absorption and endogenous excretion in premature infants and older children(8, 9, 12, 32), this technique has not previously been applied to studies in full-term infants. Unlike Fe and Zn, the average Ca intake of our study infants from beikost was minimal relative to the breast milk Ca intake. The fractional absorption of Ca from the beikost in the infants' diet is uncertain, but is likely to be less than the high levels of Ca absorption observed from human milk. Therefore, it appears that human milk was the principal source of retained Ca in the study subjects, which was not the case for Fe.
Although there are no previous stable isotopic Ca absorption studies in older infants, in a review of previous data, Hillman et al.(1) estimated dietary intakes and used balanced data from 4-mo-old infants to calculate that approximately 158 mg/d Ca would be retained by 6-mo-old infants fed a mixed diet. This calculation was made using higher values for milk Ca content and total milk volume than were used in this study. Using estimates for milk Ca concentration (0.25 mg/mL)(18) and endogenous fecal Ca excretion (3 mg/kg/d) (S. A. Abrams, unpublished data), we calculate that 68 ± 38 mg/d Ca was retained by the subjects in this study from human milk. This value is below the net retention estimate by Hillman, but comparable to the recent estimate of 52 mg/d Ca accretion between 4 and 12 mo of age by Fomon and Nelson(2). It is also similar to the mean Ca balance of 66 mg/d calculated by Matkovic(33) from data collected on two infants fed various diets providing an average of 626 mg/d Ca in the first year of life. Whether enhanced Ca retention would benefit long-term bone mineral accretion and achievement of peak mineral mass is unknown and would require long-term comparative studies.
We have previously reported Ca kinetic values for premature infants, children age 4-17 y of age, and adults(8, 17). Very few Ca kinetic data are available on healthy, full-term children during the first 4 y of life(34). Although the IDPM we calculated from 24-h pooled urine samples for the subjects in this study is not identical to Ca body pool masses determined by 5-d kinetic studies, it may provide an estimate of the size of the rapidly turning over metabolic pool of Ca(17, 34). In 74 female subjects aged 4-17 y, we previously reported a linear regression equation (r = 0.66) between IDPM and chronologic age with an intercept (value of the IDPM at birth) of 161 mg/kg pool (156 mg/kg at age 6 mo)(17). This is virtually identical to the value for the subjects in this study (170 mg/kg), suggesting a relatively linear dropoff in the mass of rapidly turning over bone during the first 17 y of life. The smaller size of the IDPM (on a body weight basis) in 5-7-mo-old infants than premature infants is consistent with a slowing of bone formation during this period relative to that achieved by premature infants or by fetuses during the third trimester of pregnancy.
Conclusions. We have used six stable isotopes of minerals to assess Ca, Zn, and Fe absorption in older, human milk-fed infants. Our results demonstrate that Fe status and Fe intake from beikost are inversely related to Fe absorption from Fe given apart from breast milk, but that mineral intake from beikost is not directly related to Fe, Zn, or Ca absorption from human milk.
Abbreviations
- RBC:
-
red blood cell
- IDPM:
-
i.v. distribution pool mass
References
Hillman LS 1990 Mineral and vitamin D adequacy in infants fed human milk or formula between 6 and 12 months of age. J Pediatr 117: S134–142
Fomon SJ, Nelson SE 1993 Calcium, phosphorus, magnesium, and sulfur. In: Fomon SJ, ed. Nutrition of Normal Infants. Mosby-Year Book, St. Louis, pp 192–218
Kastenmayer P, Davidsson L, Galan P, Cherouvrier F, Hercberg S, Hurrell RF 1994 A double stable isotope technique for measuring iron absorption in infants. Br J Nutr 71: 411–424
Abrams SA, Wen J, O'Brien KO, Stuff JE, Liang LK 1994 Application of magnetic sector thermal ionization mass spectrometry to studies of erythrocyte iron incorporation in small children. Biol Mass Spectrom 23: 771–775
Abrams SA, O'Brien KO, Wen J, Liang LK, Stuff JE 1996 Absorption by 1-year-old children of an iron supplement given with cow milk or juice. Pediatr Res 39: 171–175
Friel JK, Andrews W, Alknani T, Simmons, Longerich H 1994 Absorption of zinc in premature infants (<1500 g birthweight) measured by double isotope (68Zn, 70Zn) enrichment of urine. FASEB J 8:A918.
Friel J, Naake V, Miller L, Fennessey P, Hambidge KM 1992 The analysis of stable isotopes in urine to determine the fractional absorption of zinc. Am J Clin Nutr 36: 537–554
Abrams SA, Schanler RJ, Yergey AL, Vieira NE, Bronner F 1994 Compartmental analysis of calcium metabolism in very low birth weight infants. Pediatr Res 36: 424–428
Abrams SA, Esteban NV, Vieira NE, Yergey AL 1991 Dual tracer stable isotopic assessment of calcium absorption and endogenous fecal excretion in low birth weight infants. Pediatr Res 29: 615–618
Eastell R, Vieira NE, Yergey AL, Riggs BL 1989 One-day test using stable isotopes to measure true fractional calcium absorption. J Bone Miner Res 4: 463–468
Yergey AL, Vieira NE, Hansen JW 1980 Isotope ratio measurements of urinary calcium with a thermal ionization probe in a quadrupole mass spectrometer. Anal Chem 52: 1811–1814
Hillman LS, Tack E, Covell DG, Vieira NE, Yergey AL 1988 Measurement of true calcium absorption in premature infants using intravenous 46Ca and oral 44Ca. Pediatr Res 23: 589–594
Dixon PR, Perrin RE, Rokop DJ, Maeck R, Janecky DR, Banar JP 1993 Measurement of iron isotopes in submicrogram quantities of iron. Anal Chem 65: 2125–2130
DeBievre P, Barnes IL 1985 Table of the isotopic composition of the elements as determined by mass spectrometry. Int J Mass Spectrom Ion Processes 65: 211–230
Fairwether-Tait S, Fox T, Wharf SG, Eagles J 1995 The bioavailability of iron in different weaning foods and the enhancing effect of a fruit drink containing ascorbic acid. Pediatr Res 37: 389–394
Yergey AL, Abrams SA, Vieira NE, Aldroubi A, Marini J, Sidbury JB 1994 Determination of fractional absorption of dietary calcium in humans. J Nutr 124: 674–682
O'Brien KO, Abrams SA 1994 Effects of development on techniques for calcium stable isotope studies in children. Biol Mass Spectrom 23: 357–361
Dewey KG, Finley DA, Lönnerdal B 1984 Breast milk volume and composition during late lactation (7-20 months). J Pediatr Gastroenterol Nutr 3: 713–719
Cook JD, Bothwell TH 1984 Availability of iron from infant foods. In: Steckel A (ed) Iron Nutrition in Infancy and Childhood. Vevey/Raven Press, New York, pp 119–145
Fomon SJ, Ziegler EE, Nelson SE, Serfass RE, Frantz JA 1995 Erythrocyte incorporation of iron by 56-day-old infants fed a58 Fe-labeled supplement. Pediatr Res 38: 373–378
Davidsson L, Kastenmayer P, Yuen M, Lönnerdal B, Hurrell RF 1993 Influence of lactoferrin on iron absorption from human milk in infants. Pediatr Res 35: 117–124
Saarinen UM, Siimes MA, Dallman PR 1977 Iron absorption in infants: high bioavailability of breast milk iron as indicated by the extrinsic tag method of iron absorption and by the concentration of serum ferritin. J Pediatr 91: 36–39
Saarinen UM, Siimes MA 1979 Iron absorption from breast milk, cow's milk and iron supplemented formula: an opportunistic use of changes in total body iron determined by hemoglobin, ferritin, and body weight in 132 infants. Pediatr Res 13: 143–147
Fomon SJ 1993 Iron. In: Fomon SJ (ed) Nutrition of Normal Infants. Mosby-Year Book, St. Louis, pp 239–260
Cook JD, Dassenko SA, Whittaker P 1991 Calcium supplementation: effect on iron absorption. Am J Clin Nutr 53: 106–111
Hallberg L, Rossander-Hulten L, Brune M, Gleerup A 1992 Calcium and iron absorption: mechanism of action and nutritional importance. Eur J Clin Nutr 46: 317–322
Atkinson SA, Shah JK, Webber CE, Gibson IL, Gibson RS 1993 A multi-element isotopic tracer assessment of true fractional absorption of minerals from formula with additives of calcium, phosphorus, zinc, copper and iron in young piglets. J Nutr 123: 1586–1593
Davidsson L 1994 Minerals and trace elements in infant nutrition. Acta Paediatr Suppl 395: 36–42
Ziegler EE, Serfass RE, Nelson SE, Figueroa-Colon R, Edwards BB, Houk RS, Thompson JJ 1989 Effect of low zinc intake on absorption and excretion of zinc by infants studied with 70Zn as extrinsic tag. J Nutr 119: 1647–1653
Krebs NF, Miller LV, Naake VL, Lei S, Westcott JE, Fennessey PV, Hambidge KM 1995 The use of stable isotope techniques to assess zinc metabolism. J Nutr Biochem 6: 292–301
Sievers E, Oldigs H-D, Dörner K, Schaub J 1992 Longitudinal zinc balances in breast-fed and formula-fed infants. Acta Paediatr 81: 1–6
Abrams SA, Sidbury JB, Muenzer A, Esteban NV, Vieira NE, Yergey AL 1991 Stable isotopic measurement of endogenous fecal calcium excretion in children. J Pediatr Gastroenterol Nutr 12: 469–473
Matkovic V 1991 Calcium metabolism and calcium requirements during skeletal modeling and consolidation of bone mass. Am J Clin Nutr 54: 245S–260S
Abrams SA, Esteban NV, Vieira NE, Sidbury JB, Specker BL, Yergey AL 1992 Developmental changes in calcium kinetics in children assessed using stable isotopes. J Bone Miner Res 7: 287–293
Acknowledgements
The authors thank the nursing staff of the Metabolic Research Unit of the Children's Nutrition Research Center for nursing care of the study subjects, Lily Liang for technical assistance, Barbara Kertz for study coordination, and Leslie Loddeke for editorial assistance.
Author information
Authors and Affiliations
Additional information
Supported in part with federal funds from the USDA/ARS under Cooperative Agreement 58-6250-1-003. This work is a publication of the U.S. Department of Agriculture (USDA)/Agricultural Research Service (ARS) Children's Nutrition Research Center, Department of Pediatrics, Baylor College of Medicine and Texas Children's Hospital, Houston, TX. The contents of this publication do not necessarily reflect the views or policies of the USDA, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
Rights and permissions
About this article
Cite this article
Abrams, S., Wen, J. & Stuff, J. Absorption of Calcium, Zinc, and Iron from Breast Milk by Five- to Seven-Month-Old Infants. Pediatr Res 41, 384–390 (1997). https://doi.org/10.1203/00006450-199703000-00014
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/00006450-199703000-00014
This article is cited by
-
Bone metabolism and osteoporosis during pregnancy and lactation
Archives of Osteoporosis (2022)
-
Is early-life iron exposure critical in neurodegeneration?
Nature Reviews Neurology (2015)
-
Total calcium absorption is similar from infant formulas with and without prebiotics and exceeds that in human milk-fed infants
BMC Pediatrics (2012)