Abstract
Mental retardation associated with hypothyroidism may be caused by impairment of brain ketone body-metabolizing enzymes during the suckling period. However, much evidence suggests that, immediately after delivery, lactate, instead of ketone bodies or glucose, may be the best substrate for the brain. In this work, we have studied the effect of experimentally induced congenital hypothyroidism on the rate of lactate, glucose, and 3-hydroxybutyrate utilization in early neonatal brain slices. Methimazole(MMI) administration to the mothers caused a 5.4- and 1.7-fold decrease in neonatal plasma concentrations of L-thyroxine (T4) and 3,5,3′-triiodo-L-thyronine (T3), respectively. Propylthiouracil(PTU) administration to the mothers caused a 7.3- and >2-fold decrease in plasma T4 and T3 concentrations, respectively. MMI-induced hypothyroidism did not significantly modify the rate of lactate, glucose, or 3-hydroxybutyrate oxidation to CO2 and their incorporation into lipids by the neonatal brain. However, PTU-induced hypothyroidism decreased the rate of lactate and glucose oxidation to CO2 and their incorporation into lipids by 17% (p < 0.05). 3-Hydroxybutyrate utilization was not modified by this treatment. Separation by HPLC of the lipids revealed that PTU-mediated inhibition of lipid synthesis from lactate and glucose may be accounted for by specific inhibition of the rate of sterol synthesis (15%,p < 0.05), whereas the rate of phospholipid synthesis was unaffected. These results suggest that the early newborn may develop mechanisms aimed at avoiding the possible brain damage caused by the inhibition of lipid synthesis brought about by mild neonatal hypothyroidism.
Similar content being viewed by others
Main
Thyroid function in the rat is not instilled until late gestation, when the concentrations of T4 and T3 in plasma(1) and in brain(2, 3) show a significant increase. These events coincide with the enhancement in the activity of brain 5′D-II(4), which is the enzyme responsible for the conversion of T4 to T3. The CNS is markedly dependent on thyroid hormones for its overall growth and its biochemical and morphologic development(2, 5, 6). T4 and T3, the major thyroid hormones, regulate brain development and maturation through the binding to the T3 nuclear receptor(7), which is present in rat brain since early pregnancy(8). This is supported by earlier studies showing that in the rat thyroid hormone deficiency delayed the biochemical development of the brain during the suckling period(9, 10).
Thyroid status regulates the metabolism of lipids in the rat because postnatally induced hypothyroidism depresses lipogenesis in the developing brain and liver(11). Patel(12) showed that the delay in brain maturation caused by congenital hypothyroidism is accompanied by a decrease in the activity of the enzymes responsible for the oxidation of pyruvate and ketone bodies.
During the suckling period the brain utilizes glucose and ketone bodies as the main metabolic substrates(13). However, a number of observations are consistent with the hypothesis that lactate is also an important metabolic substrate for the brain during the early neonatal period in several species, including man (for a review, seeRef. 14). Thus, the rate of lactate utilization by fetal(15) and neonatal rat brain slices(16), isolated brain cells(17, 18), or neurons and astrocytes(19) in primary culture is much higher than that of glucose or 3-hydroxybutyrate, suggesting that immediately after delivery lactate is preferred to glucose or ketone bodies as a brain fuel. In addition, lactate transport into the brain is higher during the perinatal period than in the adult stage(20, 21), suggesting that lactate may be used by the brain throughout the perinatal period. In agreement with this, Dombrowski et al.(22) have shown that lactate may also be an important substrate for the brain during the early suckling period. The prevalence of lactate utilization over other substrates during the early neonatal period has been considered as an adaptive mechanism aimed at protecting against the brain damage caused by the lack of glucose availability in this important period(23). Therefore, the influence of congenital hypothyroidism on the ability of the early neonatal brain to use lactate may be of relevance for normal development of the newborn.
In view of the above considerations, the aim of the present work was to investigate the role of experimentally induced congenital hypothyroidism on the rate of lactate, glucose, and 3-hydroxybutyrate utilization by the early neonatal rat brain. The effect of hypothyroidism on the metabolic fate of these substrates also has been investigated.
METHODS
Reagents. L-[U-14C]Lactate (177 Ci/mol), D-[6-14C]glucose (55.8 Ci/mol), and D-3-hydroxy-[3-14C]butyrate(44.3 Ci/mol) were purchased from DuPont NEN (Boston, MA). L-Lactic acid was obtained from Serva Feinbiochemica (Heidelberg, Germany). D-Glucose and DL-3-hydroxybutyrate were obtained from Sigma Chemical Co. (St. Louis, MO). Solvents for HPLC were purchased from Scharlau (Barcelona, Spain). Enzymes and coenzymes were obtained from Böehringer Mannheim (Germany).
Animals. Albino Wistar rats housed at 23 ± 2 °C with a 12-h light-dark cycle and fed on stock laboratory diet (carbohydrate 58.7%, protein 18.0%, fat 3.0%, and added salts and vitamins) were used for the experiments. Virgin females weighing 210-260 g were caged overnight with males. Conception was considered to occur at 0100 h and was confirmed the next morning by the presence of spermatozoa in vaginal smears.
Induction of hypothyroidism. Congenital hypothyroidism was induced by the administration of 0.02% (wt/vol) of MMI(24) or 0.05% (wt/vol) of PTU(25) in the drinking water of pregnant rats from d 14 of gestation until the day of the experiment. The drug solution was replaced by a fresh one every 2 d. These anti-thyroid drugs are known to block both maternal and fetal thyroid function(26). Untreated pregnant rats were also used as a control(euthyroid). The fetuses from either euthyroid, MMI- or PTU-treated pregnant rats were delivered at d 21.5 of gestation (21.7 d for full gestation) by rapid hysterectomy after cervical dislocation of the mother. Newborns were carefully wiped, and the umbilical cords were tied and cut. Newborns were maintained in an incubator at 37 °C with a continuous stream of water-saturated air without feeding.
Incubation of brain slices. After 1 h of extrauterine life, the newborns were decapitated, and the right hemispheres of the forebrain were removed and manually sliced with a blade in a water-saturated cabinet. The width of the slice (approximately 0.5 mm) has been shown to be sufficiently thin to allow an adequate oxygenation of the brain cells(27). Brain slices (about 70 mg of wet weight) were immediately (<1 min after decapitation) incubated as previously described(15, 16). The incubation medium was 2 mL of PBS (11 mM sodium phosphate, 122 mM NaCl, 3.1 mM KCl, 0.4 mM KH2PO4, 1.2 mM MgSO4, and 1.3 mM CaCl2) at pH 7.4. The incubation medium also contained 2 μCi of L-[U-14C]lactate, 2 μCi of D-[6-14C]glucose, or 1 μCi of D-3-hydroxy-[3-14C]butyrate and 11.9 ± 0.2 mM, 5.22 ± 0.04 mM, or 1.19 ± 0.03 mM of the unlabeled substrates, respectively. These concentrations are close to physiologic blood concentrations in the rat at this stage of development(28) and have been shown to be suitable for the study of brain metabolism in slices(15, 16, 29), isolated cells(17, 18), or neurons and astrocytes in primary culture(19). The flasks were gassed for 30 s with pure oxygen (this was enough to maintain high Po2 in the incubation medium throughout the experiment), sealed with rubber caps, and incubated in a shaking water bath at 37 °C.
Measurement of the rate of substrate oxidation to CO2. Incubations were stopped after 2 h by injection of 0.2 mL of 4.75 M HClO4 through the rubber cap into the main well, although shaking was continued for an additional 20 min to facilitate the trapping of CO2. The 14CO2 evoked by the slices was trapped by 3.56 M KOH placed in a center well, and the radioactivity was measured by liquid scintillation counting (LS 1800; Beckman, Palo Alto, CA). Blanks without slices were carried out in parallel to measure background radioactivity, which was subtracted from the sample values. This method has been validated previously, the 14CO2 evoked by the brain slices being proportional to the weight of tissue in the 40-100 mg/flask range and to the incubation period(30).
Extraction of total lipids. At the end of the incubations, the slices were frozen under liquid nitrogen. Total lipids were extracted from the powdered tissue with 2 mL of a mixture of chloroform:methanol (2:1, vol/vol) as in the method described by Folch et al.(31) for 16 h at -20 °C. The extract was washed twice with 0.8 mL of 0.3%(wt/vol) NaCl saturated with chloroform. The organic phase was divided into two aliquots; 0.1 mL was used for the measurement of the radioactivity incorporated into total lipids, and 1.0 mL was gently dried under a stream of N2 and kept at -20 °C until subjected to phospholipid separation by HPLC(15).
Separation of phospholipid and sterols by HPLC. Phospholipid species were separated by an HPLC isocratic method using a normal phase column of silica (Ultrasphere, Beckman) coupled to a pump delivery system (114 M-Beckman) and to a UV detector (163-Beckman) with an eluent mixture of acetonitrile:methanol:9.79 M H2SO4 (100:3:0.052, vol/vol/vol) at a flow rate of 1 mL/min as previously described(15). Phospholipid species. i.e. phosphatidylcholine, phosphatidylethanolamine, phosphatidylinositol, and phosphatidylserine were separated and collected (0.5-mL fractions) in scintillation vials using a fraction collector (2110 Bio-Rad, Richmond, CA) coupled to the detector output, and the radioactivity was counted. Sterols ran together with the solvent front, which was verified by thin layer chromatography (Silica Gel G-200, Merck, Darmstad, Germany), using a system of chloroform: acetone (95:5, vol/vol) as the mobile phase(15).
Measurement of plasma concentrations of thyroid hormones. Blood samples were obtained from the severed necks of euthyroid and MMI- and PTU-treated newborns using heparinized capillary tubes. Plasma from five newborns of the same litter were pooled and considered as a sample. Plasma T4 was measured using a kit consisting of a solid-phase time-resolved fluoroimmunoassay method (DELFIA thyroxine, Pharmacia, Wallac Oy, Finland). Plasma T3 was measured by the solid phase enzyme immunoassay kit(Enzymon-test T3, Boehringer Mannheim) in a automated enzyme immunoassay system (ES-600).
Analytical procedures. L-Lactate was measured as described by Gutmann and Wahlefeld(32), D-glucose as reported by Bergmeyer et al.(33), and D-3-hydroxybutyrate as reported by Williamson and Mellanby(34). The specific radioactivity of the substrates found in the blanks was used for the calculations. The rates of substrate utilization by the brain slices were expressed as micromoles (or nanomoles) of L-lactate, D-glucose, or D-3-hydroxybutyrate oxidated to CO2 or incorporated into total lipids, sterols, or phospholipids/h/g of wet weight. Results are means ± SEM. Statistical analyses of the results were carried out by one-way analysis of variance followed by a least significant difference multiple rage test.
RESULTS
Effect of MMI or PTU treatment on plasma thyroid hormone concentrations of newborn rats. Treatment with MMI decreased the T4 and T3 plasma concentrations by 5.4- and 1.7-fold, respectively, as compared with euthyroid newborns. PTU treatment decreased T4 plasma concentrations by 7.3-fold, and T3 levels reached values below the sensitivity of the method (<0.3 ng/mL). In addition, neonatal body weight(Table 1) significantly decreased compared with euthyroid newborns (p < 0.001) after treatment with both MMI and PTU.
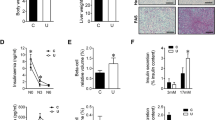
Effect of congenital hypothyroidism on substrate utilization by early neonatal brain. Figure 1 shows the rate of lactate, glucose, and 3-hydroxybutyrate oxidation to CO2 and their incorporation into total lipids by the neonatal brain in euthyroid and in hypothyroid (MMI and PTU) animals. The rate of lactate oxidation to CO2 (Fig. 1a) was about 10- and 4-fold higher than those found for glucose (Fig. 1c) or 3-hydroxybutyrate (Fig. 1b), respectively. Likewise, lactate incorporation into total lipids (Fig. 1a) was about 1.4- and 5-fold higher than that found from glucose (Fig. 1c) or 3-hydroxybutyrate (Fig. 1b), respectively. MMI-induced hypothyroidism did not significantly change either the rate of oxidation or lipogenesis from the three substrates assayed. However, PTU-induced hypothyroidism caused a significant decrease (17%, p < 0.05) in the rates of lactate (Fig. 1a) and glucose (Fig. 1c) utilization, both by oxidation and by lipogenesis. 3-Hydroxybutyrate utilization was unchanged by PTU treatment (Fig. 1b).
Effect of congenital hypothyroidism on lactate, 3-hydroxybutyrate, and glucose utilization by newborn rat brain. Brain slices from early (1-h-old) newborns were incubated at 37 °C in phosphate buffer(pH 7.4) containing 2 μCi of L-[U-14C]lactate, 2 μCi of D-[6-14C]glucose, or 1 μCi of D-3-hydroxy-[3-14C]butyrate and 11.9 ± 0.2 mM, 5.22 ± 0.04 mM, or 1.19 ± 0.03 mM of the unlabeled substrates, respectively. The rates of substrate oxidation to CO2 and its incorporation into lipids were determined as described in“Methods.” Results are means ± SEM for 17 newborns in euthryroid-treated group (EU), 10 newborns in MMI-treated group(MMI), and 10 newborns in PTU-treated group (PTU).*p < 0.05 compared to euthyroid; #p < 0.05 compared with methimazole.
Effect of congenital hypothyroidism on the rate of substrate incorporation into phospholipid species and into sterols in early neonatal brain. Table 2 shows that lactate was the main precursor of sterols and phospholipids compared with glucose and 3-hydroxybutyrate, phosphatidylcholine being the main phospholipid species synthesized from lactate, glucose, or 3-hydroxybutyrate(Table 2). MMI-induced hypothyroidism did not change the incorporation of these substrates into sterols or phospholipid species(Table 2). However, PTU-induced hypothyroidism decreased the incorporation of lactate and glucose into sterols by 15% (p < 0.05), without modifying their incorporation into phospholipid species(Table 2).
DISCUSSION
The rates of substrate utilization by the brain of euthyroid rats observed in our experiments are consistent with those found in previous studies using brain slices(15, 16, 29), isolated brain cells(17, 18), and cultured neurons and astrocytes(19, 35). This provides further evidence showing that lactate is an important substrate for the early newborn rat brain.
PTU-induced hypothyroidism caused a significant decrease in the rates of lactate and glucose utilization by neonatal brain, although these changes were not observed in MMI-induced hypothyroid newborns (Fig. 1). These results were not unexpected because the concentration of thyroid hormones were significantly lower in PTU-treated newborns compared with MMI-treated newborns (Table 1). The maintenance of intracellular concentrations of T3 in the neonatal brain mainly depends on the in situ conversion of T4 to T3 brought about by 5′D-II. Although type II isoezyme is not inhibited by PTU(36), it does use plasma T4 as substrate, this latter being the main source of T3 for the brain(37, 38). Our results show that plasma T4 concentrations were significantly lower in PTU-treated newborns than in MMI-treated newborns (Table 1), suggesting a decreased availability of T4 for the brain of the PTU-treated newborns. In addition, another putative source of T3 for the brain is plasma T3, whose concentrations were significantly lower in PTU-treated newborns than those found in euthyroid or MMI-treated newborns(Table 1). This may be a consequence of a decreased hepatic monodeiodination, because liver 5′-deiodinase type I,i.e. the major source of plasma T3, is strongly inhibited by PTU(36, 39, 40). Our results are therefore consistent with the idea that the rates of lactate and glucose utilization by neonatal brain are affected only when T3 availability is seriously reduced. In agreement with this, PTU-induced, but not MMI-induced, hypothyroidism decreased the incorporation of lactate and glucose into sterols(Table 2). Therefore, the incorporation of these substrates into phospholipid species was not changed by hypothyroidism(Table 2), suggesting that the inhibition of lipogenesis observed in the brains of hypothyroid newborns (Fig. 1) may be due to a specific impairment of the synthesis of sterols(Table 2). Again, the effect was observed in PTU-treated but not in MMI-treated animals, suggesting that T3 would be directly involved in this phenomenon. On the other hand, a direct effect of PTU on brain metabolism can be ruled out because the presence of PTU in the incubation medium at a concentration similar to that used in the drinking water (0.05%, wt/vol) did not affect lactate or glucose utilization by neonatal brain (results not shown).
Some evidence has been reported(3, 24, 37, 41, 42) supporting the notion that fetal and neonatal rat brain would develop some mechanisms focused on mitigating the possible damage caused by congenital hypothyroidism. Thus, during the neonatal period the supply of T3 to the brain is accomplished mainly by the conversion of plasma T4 into T3 through the type II 5′-deiodinase-catalyzed reaction(37, 38). The activity of this enzyme in the brain starts to increase before the onset of fetal thyroid function(3) and is up-regulated thereafter by low thyroid hormone levels(3, 24, 43). Moreover, it is known that in neonatal brain hypothyroidism markedly decreases the rate of T4-inner ring 5-deiodination, by which T4 is diverted to the synthesis of inactive reverse-T3 (3,3′,5′-triiodothyronine)(36, 44). Because this inhibition presumably increases T4 availability for T3 synthesis, it may be considered as a mechanism designed to prevent brain T3 depletion under hypothyroidism. Consequently, it is reasonable to suggest that both mechanisms may operate in our experimental conditions to ensure that metabolic substrates, such as lactate and glucose, can be used by the brain of MMI-treated newborns (Fig. 1) despite the low levels of circulating thyroid hormones observed under these circumstances(Table 1).
The fact that PTU-induced hypothyroidism inhibited lactate and glucose but not 3-hydroxybutyrate utilization by the newborn brain (Fig. 1) is intriguing. Thus, it has been reported that neonatal hypothyroidism induced by radiothyroidectomy strongly decreases the activity of ketone body-metabolizing enzymes such as 3-hydroxybutyrate dehydrogenase (EC 1.1.1.30), 3-oxo acid CoA-transferase (EC 2.8.3.5), and acetoacetyl-CoA thiolase (EC 2.3.1.9) in the brain from 2-4-wk-old rats(12). However, it should be mentioned that the same authors(12) were unable to find any effect of hypothyroidism on the activities of these enzymes in early suckling (1-wk-old) rats, suggesting that immediately after birth ketone bodies utilization by the brain is resistant to hypothyroidism. On the other hand, under the same circumstances pyruvate dehydrogenase complex (EC 1.2.4.1 + EC 2.3.1.12 + EC 1.8.1.4) activity is decreased by hypothyroidism(12), which may explain the different effect of PTU-induced hypothyroidism on 3-hydroxybutyrate utilization compared with the effects on glucose and lactate utilization observed in our experiments (Fig. 1). Indeed, 3-hydroxybutyrate is cleaved into acetyl-CoA in the cytosol by a brain exclusive pathway(13), but pyruvate from lactate or glucose is compulsorily transformed into acetyl-CoA within the mitochondria, which is later transported to the cytosol in the form of citrate. It is therefore tempting to speculate that the observed inhibition of lactate and glucose utilization caused by severe PTU-induced hypothyroidism may be brought about by the decrease in the pyruvate dehydrogenase activity caused by thyroid hormone deprivation(12). This mechanism also explains the lack of effect on 3-hydroxybutyrate utilization under our experimental conditions (Fig. 1), because pyruvate dehydrogenase is not required for ketone body utilization in the newborn brain(13).
In conclusion, our results suggest that immediately after birth newborn brain shows a resistance to the effects of thyroid hormone deprivation on the utilization of the main metabolic substrates for the brain such as lactate, glucose, and 3-hydroxybutyrate.
Abbreviations
- 5′D-II:
-
iodothyronine 5′-deiodinase type II
- MMI:
-
methimazole
- PTU:
-
propylthiouracil
- T4:
-
L-thyroxine
- T3:
-
3,5,3′-triiodo-L-thyronine
References
Morreale de Escobar G, Pator R, Obregon MJ, Escobar del Rey F 1985 Effects of maternal hypothyroidism on the weight and thyroid hormone content of rat embryonic tissues, before and after onset of fetal thyroid function. Endocrinology 117: 1890–1900
Morreale de Escobar del Rey F, Ruiz-Marcos A 1983 Thyroid hormone and the developing brain. In: Dussault JH, Walker P (eds) Congenital Hypothyroidism. Marcell Dekker, New York, pp 85–126
Ruiz de Oña C, Obregón MJ, Escobar del Rey F, Morreale de Escobar G 1988 Developmental changes in rat brain 5′-deiodinase and thyroid hormones during the fetal period: the effects of fetal hypothyroidism and maternal thyroid hormones. Pediatr Res 24: 588–594
Ruiz de Oña C, Morreale de Escobar G, Calvo R, Escobar del Rey F, Obregón MJ 1991 Thyroid hormones and 5′-deiodinase in the rat fetus late in gestation: effects of maternal hypothyroidism. Endocrinology 128: 422–432
Eayrs JT 1960 Developmental relationship between brain and thyroid. In: Michael RP (ed) Endocrinology and Human Behaviour. Oxford University Press, London, pp 239–255
Ford DH, Cramer EB 1977 Developing nervous system in relation to thyroid hormones. In: Grave GD (ed) Thyroid Hormone and Brain Development. Raven Press, New York, pp 1–18
Timiras PS, Nzekwe EU 1989 Thyroid hormones and nervous system development. Biol Neonate 55: 376–385
Pérez-Castillo A, Bernal J, Ferreiro B, Pans T 1985 The early ontogenesis of thyroid hormone receptor in the rat fetus. Endocrinology 117: 2457–2461
Balazs R, Brooksbank BWL, Davidson AN, Eayrs JT, Wilson DA 1969 The effect of neonatal thyroidectomy on myelination in the rat brain. Brain Res 15: 219–232
Pharoah POD, Buttfield IH, Hetzel BS 1971 Neurological damage to the fetus resulting from severe iodine deficiency during pregnancy. Lancet 1: 308–310
Hoch F 1988 Lipids and thyroid hormones. Prog Lipid Res 27: 199–270
Patel MS 1979 Influence od neonatal hypothyroidism on the development of ketone-body-metabolizing enzymes in rat brain. Biochem J 184: 169–172
Robinson AM, Williamson DH 1980 Physiological roles of ketone bodies as substrates and signals in mammalian tissues. Physiol Rev 60: 143–187
Medina JM, Vicario C, Juanes M, Fernández E 1992 Biochemical adaptations to early extrauterine life. In: Herrera E, Knopp R(eds) Perinatal Biochemistry. CRC Press, Boca Raton, FL, pp 233–258
Bolaños JP, Medina JM 1993 Lipogenesis from lactate in fetal rat brain during late gestation. Pediatr Res 33: 66–71
Fernández E, Medina JM 1986 Lactate utilization by the neonatal rat brain in vitro: competition with glucose and 3-hydroxybutyrate. Biochem J 234: 489–492
Vicario C, Arizmendi C, Malloch G, Clark JB, Medina JM 1991 Lactate utilization by isolated cells from early neonatal rat brain. J Neurochem 57: 1700–1707
Vicario C, Medina JM 1992 Metabolism of lactate in the rat brain during the early neonatal period. J Neurochem 58: 32–40
Vicario C, Tabernero A, Medina JM 1993 Regulation of lactate metabolism by albumin in rat neurons and astrocytes from primary culture. Pediatr Res 34: 709–715
Cremer JE, Cunningham VJ, Pardridge WM, Braun LD, Oldendorf WH 1979 Kinetics of blood-brain barrier transport of pyruvate, lactate and glucose in suckling, weanling and adult rats. J Neurochem 33: 439–445
Siesjö BK 1988 Hypoglycemia, brain metabolism, and brain damage. Diabetes Metab Rev 4: 113–144
Dombrowski GJ Jr, Swiatek KR, Chao KL 1989 Lactate, 3-hydroxybutyrate and glucose as substrates for the early postnatal rat brain. Neurochem Res 14: 667–675
Medina JM, Vicario C, Bolaños JP, Tabernero A, Fernández E 1993 Lactate utilization and neonatal brain vulnerability. In: Medina JM, Quero J (eds) Physiologic Basis of Perinatal Care. Ediciones Ergon SA, Madrid, pp 187–201
Silva JE, Larsen PR 1982 Comparison of iodothyronine 5′-deiodinase and other thyroid-hormone-dependent enzyme activities in the cerebral cortex of hypothyroid neonatal rat: evidence for adaptation to hypothyroidism. J Clin Invest 70: 1110–1123
Hahn P 1986 Effect of hypothyroidism during late gestation and in the sucking period on cholesterol and carnitine metabolism in the rat. Biol Neonate 50: 259–264
Marchant B, Brownlie BEW, McKay HD, Horton PW, Alexander WD 1977 The placental transfer of propylthiouracil, methimazole and carbimazole. J Clin Endocrinol Metab 45: 1187–1192
Itoh T, Quastel JH 1970 Acetoacetate metabolism in infant and adult rat brain in vitro. Biochem J 116: 641–655
Girard GR, Cuendet GS, Marliss EB, Kervran A, Rieutort M, Assan R 1973 Fuels, hormones and liver metabolism at term and during the early postnatal period in the rat. J Clin Invest 52: 3190–3200
Bolaños JP, Medina JM 1993 Effect of valproate on lipogenesis in neonatal rat brain. Biochem Pharmacol 45: 1283–1288
Arizmendi C, Medina JM 1983 Lactate as an oxidizable substrate for rat brain in vitro during perinatal period. Biochem J 214: 633–635
Folch J, Lees M, Sloane-Stanley GH 1957 A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem 226: 497–509
Gutmann I, Wahlefeld AW 1974 L-(+)-Lactate: determination with lactate dehydrogenase and NAD. In: Bergmeyer HU (ed) Methods of Enzymatic Analysis. Vol 3. Verlag Chemie GmbH, Weinheim, pp 1464–1468
Bergmeyer HU, Bernt E, Schmidt F, Stork H 1974 D-Glucose. Determination with hexokinase and glucose-6-phosphate dehydrogenase. In: Bergmeyer HU (ed) Methods of Enzymatic Analysis, Vol. 3. Verlag Chemie GmbH, Weinheim, Germany. pp 1196–1201
Williamson DH, Mellanby J 1974 D-(-)-3-Hydroxybutyrate. In: Bergmeyer HU (ed) Methods of Enzymatic Analysis, Vol 4. Verlag Chemie GmbH, Weinheim, Germany, pp 1836–1839
Tabernero A, Bolaños JP, Medina JM 1993 Lipogenesis from lactate in rat neurons and astrocytes in primary culture. Biochem J 294: 635–638
Silva JE, Mathews PS 1984 Production rates and turnovers of triiodothyronine in rat developing cerebral cortex and cerebellum: response to hypothyroidism. J Clin Invest 74: 1035–1049
Obregón MJ, Larsen PR, Silva JE 1986 The role of 3:5,3′-triiodothyronine in the regulation of type II iodothyronine 5′-deiodinase in the rat cerebral cortex. Endocrinology 119: 2186–2192
Morreale de Escobar G, Obregon MJ, Escobar del Rey F 1987 Fetal and maternal thyroid hormones. Hormone Res 26: 12–27
Kaplan MM, Utiger RD 1978 Iodothyronine metabolism in rat liver homogenates. J Clin Invest 61: 459–471
Obregón MJ, Larsen PR, Silva JE 1985 Plasma kinetics, tissue distribution and cerebrocortical sources of reverse triiodothyronine in the rat. Endocrinology 2192: 2200
Silva JE, Leonard JL 1985 Regulation of rat cerebrocortical and adenohypophyseal type II 5′-deiodinase by thyroxine, triiodothyronine and reverse triiodothyronine. Endocrinology 116: 1627–1635
Morreale de Escobar G, Obregon MJ, Ruiz de Oña C, Escobar del Rey F 1988 Transfer of thyroxine from the mother to the rat fetus near term: effects on brain 3:5,3′-triiodothyronine deficiency. Endocrinology 122: 1521–1531
Morreale de Escobar G, Obregón MJ, Ruiz de Oña C, Escobar del Rey F 1989 Comparison of maternal to fetal transfer of 3:5,3′-triiodothyronine versus thyroxine in rats, as assessed from 3,5,3′-triiodothyronine levels in fetal tissues. Acta Endocrinol 120: 20–30
Huang TS, Chopra IJ, Boado R, Solomon DH, Teco GNC 1988 Thyroxine inner ring monodeiodinating activity in fetal tissues of the rat. Pediatr Res 23: 196–199
Acknowledgements
The authors are grateful to J. Villoria and to T. del Rey for their technical assistance.
Author information
Authors and Affiliations
Additional information
Supported by CICYT and FISSS. A.A. was the recipient of a Acción de Reincorporación del MEC, Spain.
Rights and permissions
About this article
Cite this article
Almeida, A., González-Buitrago, J., Bolaños, J. et al. Fuel Utilization by Early Newborn Brain Is Preserved under Congenital Hypothyroidism in the Rat. Pediatr Res 40, 410–414 (1996). https://doi.org/10.1203/00006450-199609000-00008
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/00006450-199609000-00008