Abstract
We investigated whether leakage of protein in lungs of preterm ventilated rabbits of 28- and 29-d gestational age is correlated with activation of clotting, complement, and polymorphonuclear leukocytes (PMN) in plasma. We found signs of systemic activation of clotting, complement, and PMN in ventilated 28-d gestational age rabbits, as indicated, respectively, by increased median plasma fibrin monomer concentrations (83 versus 40% of normal adult rabbit plasma in nonventilated 28-d gestational age rabbits,p < 0.01), decreased median plasma CH50 activity (112versus 122 U/L in nonventilated 28-d gestational age rabbits,p < 0.05), and increased median plasma β-glucuronidase concentrations (159 versus 97% of maximal activated adult rabbit plasma in nonventilated 28-d gestational age rabbits, p < 0.05). We did not find signs of systemic activation in the ventilated 29-d gestational age group. Higher median total protein concentrations in alveolar wash of the ventilated 28-d gestational age rabbits (2.7 versus 1.3 mg/mL in the nonventilated rabbits, p < 0.01) indicated protein leakage into the lungs, and this protein leakage was more pronounced in the lungs of ventilated 28-d gestational age rabbits than in those of ventilated 29-d gestational age rabbits (2.1 mg/mL, p < 0.01). The total protein concentration in the alveolar wash of all 28-d gestational age rabbits was correlated with the concentration of fibrin monomers (ρ = 0.51,p = 0.035) and β-glucuronidase (ρ = 0.61, p = 0.011), and the CH50 activity (ρ = -0.73, p = 0.002) in plasma. We conclude that leakage of protein in lungs of preterm ventilated rabbits of 28-d gestational age is correlated with activation of clotting, complement, and PMN in plasma. This activation process may contribute to lung injury by intravascular and intraalveolar deposition of fibrin and formation of proteinaceous edema.
Similar content being viewed by others
Main
Increased permeability of the alveolar-capillary membrane is a characteristic feature of the neonatal RDS in preterm infants(1). This increased permeability allows protein leakage into and out of the alveoli and small airways within a few hours of birth, as has been demonstrated in preterm ventilated animals(2–5). During the first few hours of life, protein moves predominantly into the lungs(3–6), thus causing formation of interstitial and intraalveolar protein-rich edema(7). Interstitial edema decreases lung compliance and impairs gas exchange. Intraalveolar edema contains plasma proteins, which contribute to the formation of hyaline membranes(8) and inactivation of surfactant(9–12). Surfactant inactivation leads to alveolar collapse and impairs lung function(13).
The increased permeability of the alveolar-capillary membrane is thought to be dependent on mechanical forces that damage lung tissue and occurs during artificial ventilation or even spontaneous breathing(3, 14, 15). In preterm lambs, however, the amount of protein leakage increases with decreasing gestational age, while ventilating them with equivalent peak inspiratory pressures(4). An increase of protein leakage was also demonstrated in unventilated segments of preterm lamb lungs(16). Therefore, the increased permeability of the alveolar-capillary membrane in preterm lungs is suggested to be primarily dependent on the degree of immaturity and to be aggravated by barotrauma. Furthermore, activated plasma proteins and cells may contribute to this increased permeability.
Systemic activation of plasma protein systems (clotting, fibrinolysis, kinin-kallikrein, complement) and cells (leukocytes, platelets) with subsequent release of bioactive mediators has been described in severely ill children and adults with adult respiratory disease and multiple organ failure. This systemic activation process is directed against host tissues, especially the lungs(17, 18). Recently, activation of clotting, fibrinolysis, kinin-kallikrein, and complement has been described in preterm infants with severe RDS during the first few days of life(19–22). Products of these activated plasma protein systems are able to injure pulmonary endothelium directly or indirectly by activation of platelets and PMN(17, 18), which may contribute to protein-rich edema formation and disease severity in RDS. So far, a positive correlation has been observed between clotting activation and disease severity in 3-d-old preterm infants with neonatal RDS(19). However, the correlation between leakage of protein into the small airways and activation of plasma proteins and cells has not been studied in neonatal RDS. Also, it is not known whether activation of circulating PMN occurs in neonatal RDS, because activation of PMN has been studied only in tracheal effluent by measuring concentrations of PMN release products such as elastase(23–27). We, therefore, studied simultaneously the activation of clotting (fibrin monomers), complement(CH50), and PMN (β-glucuronidase) in plasma and the total protein concentration in bronchoalveolar lavage fluid of preterm rabbits of 28 and 29 d gestational age, that were not ventilated or ventilated for 30 or 60 min.
METHODS
Animals. The experiments in this study were performed under approved animal care protocols of the University of Groningen with concern for animal welfare. Because this study was undertaken to investigate the correlation between protein leakage in the small airways and clotting-complement-PMN activation in plasma, we required rabbits with different lung maturity to obtain differences in protein leakage. We decided to study preterm newborn chinchilla rabbits of 28- and 29-d gestational age(term is 31 d), because, in pilot studies, we found considerable differences between these two gestational age groups with regard to ventilatory requirements and protein leakage in the small airways. These rabbits were either artificially ventilated or not. Preterm rabbits were excluded from analysis because of 1) antenatal loss, 2) the occurrence of an air leak in the upper airways during placement of the endotracheal tube,3) the development of a pneumothorax during ventilation, or4) inadequate artificial ventilation as defined by a final Pco2 > 9.0 kPa after ventilation. Rabbits with an upper airway leak or pneumothorax were excluded from the final analysis because a proper lung lavage procedure could not be performed. Rabbits that developed a pneumothorax during ventilation showed severe circulatory insufficiency, whereas rabbits with a Pco2 > 9.0 kPa after ventilation showed severe respiratory acidosis. Both conditions are known to activate the parameters studied, and such animals were, therefore, excluded from final analysis.
Surgical preparation. On d 28 or 29 of gestation, pregnant chinchilla doe were anesthetized by slow infusion of sodium pentobarbital(Nembutal, 30 mg/kg), intubated endotracheally, and ventilated artificially(Amsterdam Infant Ventilator MK III, Hoekloos Co., Schiedam, The Netherlands). The pregnant doe underwent cesarian section after a short stabilization period on the ventilator. After delivery, preterm newborn rabbits were weighed and given 10 mg/kg ketamine by intraperitoneal injection. After performing a tracheostomy a homemade 18 gauge tube was tied into the trachea of each rabbit.
Experimental protocol. After endotracheal intubation, one out of five newborn rabbits from each litter was not ventilated (nonventilated group) and immediately killed by an intrathecal injection of lidocaine. The remaining rabbits were randomized to a ventilation period of 30 or 60 min(30-min and 60-min ventilated group). It has been shown that ventilation of preterm rabbits over a period of 30 min is long enough to find an increase of protein in the small airways(5, 28). An increase of this protein leakage has been found in preterm ventilated lambs during the first 3 h of life(3, 4). We, therefore, selected arbitrarily the time points of 30 and 60 min to determine whether a similar increase of protein leakage occurs in the ventilated rabbits and whether this is accompanied by increased activation of clotting, complement, and circulating PMN. Lungs of the ventilated rabbits were inflated five times using a small ventilation balloon fit to the tube with enough pressure to see the chest moving. Then they were placed in a 37°C temperature controlled ventilator-plethysmograph system. This system allows frequent simultaneous measurement of ventilation pressures via a water column and integrated tidal volumes using a pneumotachometer(5).
All newborn rabbits of the 30- and 60-min ventilated groups were initially ventilated with 100% oxygen, an inspiratory time of 0.5 s, a PIP of 35 cm H2O. The 28-d gestational age rabbits were ventilated at a rate of 40 breaths/min; the 29-d gestational age rabbits at a rate of 30 breaths/min(5). All rabbits were ventilated without using positive end-expiratory pressure to avoid air trapping(5). Immediately after starting artificial ventilation the PIP was adjusted individually by means of a pop-off system to achieve a tidal volume of 10-12 mL/kg. Dynamic compliance and ventilatory efficiency index values were calculated just before termination of the experiment after which all ventilated rabbits were killed by an intrathecal injection of lidocaine.
Immediately after death the chest of each rabbit was opened to obtain a heparinized blood sample (0.2 mL) and a citrated blood sample (0.8 mL) by cardiac puncture (right atrium and right ventricle). After blood sampling, a lung lavage procedure was performed according to the procedure described by Ikegami et al.(5). Five aliquots of a 0.9% saline solution (room temperature) were slowly instilled until the lungs were visibly distended. Total lung distension was achieved with approximately 1.5 mL of saline per aliquot in each rabbit. Each aliquot was three times instilled and withdrawn. The pooled volume of all aliquots per rabbit was considered to be the total alveolar wash.
Processing of the samples. The heparinized blood sample was used for measurement of Pco2 and pH; the citrated blood sample was centrifuged and stored at -80°C until values of CH50 and plasma concentrations of fibrin monomers, β-glucuronidase, and total protein were determined. Alveolar wash samples were centrifuged and stored at-80°C until determination of total protein concentrations.
Assays. For determination of pH and Pco2, a ABL 330 blood gas analyzer (Radiometer Co., Copenhagen, Denmark) was used. The fibrin monomer plasma concentration was determined using a previously described assay(29) with minor modifications. In brief, rabbit plasma(20 μL) was pipetted into a microtiter well and incubated for 2 h at 37°C with 130 μL of a plasminogen solution (0.03 mg/mL) in Tris-HCl buffer (pH 7.4) and 20 μL of a tissue plasminogen activator solution (2.5 ng/mL) and S2251 substrate (Kabi, Stockholm, Sweden). Absorbance was measured at 405 nm in a spectrophotometer before and after incubation. The change of absorbance represents the fibrin monomer plasma concentration, which was expressed as percentage substrate conversion in activated adult rabbit plasma.
The total complement hemolytic assay (CH50) was performed according to Mayer et al.(30), with sensitized sheep red blood cells (rabbit anti-sheep red blood cells, Cappel, Holland) incubated with 1:2 to 1:64 stepwise diluted rabbit serum. The β-glucuronidase plasma concentration was measured according to Beahner et al.(31). A mixture of 12.5 μL of substratep-nitrophenyl β-D-glucopyranoside uric acid solution (Merck, Darmstadt, Germany) and 25 μL of rabbit plasma was incubated for 2 h at room temperature. The conversion of the substrate by plasmaβ-glucuronidase was determined at 410 nm in a spectrophotometer(Microplate Reader 3550 UV, Bio-Rad, Richmond CA). The β-glucuronidase concentration was expressed as percentage of substrate conversion in normal adult rabbit plasma.
Total protein concentrations in plasma and alveolar washes was measured according to Lowry et al.(32). The lung lavage procedure was standardized as mentioned above. The recovered volume of pulmonary effluent by each lavage procedure did not differ between ventilation groups within each gestational age group or between similar ventilation groups of both gestational age groups. Therefore, total protein concentration of alveolar wash was expressed as milligrams/mL of pulmonary fluid.
Calculations. Compliance was calculated as total lung compliance by dividing tidal volume (mL) by PIP (cm H2O) and body weight (kg). The VEI was calculated according to Notter et al.(33) using the following equation: 3800/(ΔP × F × PaCO2), where 3800 is a constant relating to CO2 production (mL × mm Hg/kg × min), ΔP is the PIP minus the positive end-expiratory pressure(cm H2O), F is the ventilatory frequency, and Paco2 is the arterial Pco2 (mm Hg). This index estimates the overall ventilation efficiency of mechanically ventilated animals accounting for the combined effect of different ventilatory pressures, rates, and Pco2 values. The VEI increases as lung function improves.
Statistical analysis. Data are presented as mean ± SD or as median with 25th and 75th percentiles as appropriate. Within each gestational age group, the values for pH, Pco2, tidal volume, PIP, compliance, and VEI of the nonventilated and ventilated subgroups were compared with one-way analysis of variance followed by the unpairedt test with Bonferroni correction for multiple comparison. For CH50 values and β-glucuronidase, fibrin monomers, and total protein concentrations, statistical analysis was performed with the Kruskall-Wallis test followed by the Mann-Whitney U test. Adjustment of the significance level for multiple comparison was performed according to the Bonferroni correction. If no differences were found between the 30- and 60-min ventilated subgroups within each gestational age group, values were combined into a single ventilated group and compared with the nonventilated group using the unpaired t test or the Mann-Whitney U test as appropriate.
The values of the nonventilated and the two ventilated subgroups of the 28-d gestational age group were compared with those of the 29-d gestational age group by means of the unpaired t test or the Mann-WhitneyU test. Correlations were carried out by means of the Spearman rank correlation test. A p value of less than 0.05 was considered to be significant.
RESULTS
Animals. Ten litters of chinchilla rabbits of 28- and 29-d gestational age were available for this study, yielding 64 fetuses. Characteristics of these fetuses are presented in Table 1. There were three antenatal losses in the 28-d gestational age group and none in the 29-d gestational age group (not significant). The number of animals excluded and the mean birth weights of the three ventilation groups did not differ significantly between or within the two gestational age groups.
Blood gas analysis values and ventilatory characteristics of the rabbits used for analysis are presented in Table 2. The nonventilated rabbits in each gestational age group showed respiratory insufficiency as demonstrated by low mean pH values and high mean Pco2 values. The mean Pco2 values were significantly lower and the mean pH values significantly higher in the ventilated 28- and 29-d gestational rabbits regardless of ventilation time.
Mean tidal volumes were successfully maintained within the desired range necessary to ventilate rabbits in both gestational age groups properly. However, higher peak inspiratory pressures were required in the 28-d gestational age rabbits than in the 29-d gestational age rabbits. Also the mean compliance and VEI differed significantly between the ventilation subgroups of the two gestational age groups.
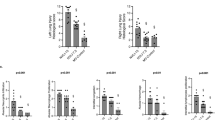
Fibrin monomers. Differences between the ventilated and nonventilated rabbits in both gestational age groups are presented inTable 3. In both gestational age groups, the fibrin monomer concentrations of the 30- and 60-min ventilated rabbits were not different and combined into one ventilation group (all ventilation). In the 28-d gestational age rabbits, the fibrin monomer concentration of the nonventilated group is significantly lower than that of the 30-min(p < 0.05) and 60-min (p < 0.05) ventilated groups separately, and than that of the all ventilated group (p < 0.01). In the 29-d gestational age rabbits, the fibrin monomer concentrations of the nonventilated and ventilated groups were not significantly different. Differences between the 28- and 29-d gestational age rabbits are presented in Figure 1.
The plasma fibrin monomer concentration of the 28-d gestational age (open boxes) and 29-d gestational age (gray boxes) rabbits, that were not ventilated (no vent.) or ventilated for 30 min (30′ vent.) or 60 min (60′ vent.). Data of the 30- and 60-min ventilated rabbits in each gestational age group were not different and combined to one single ventilation group (all vent.). Data are presented as box graphs showing the median values(horizontal plane line), ranges of 50% around the median value(boxes), and the 10th and 90th percentile (error bars).*p < 0.05; **p < 0.01 for 28-d compared with 29-d rabbits.
CH50. Differences between the ventilated and nonventilated rabbits in both gestational age groups are presented in Table 3. The fibrin monomer concentrations of the 30- and 60-min ventilated rabbits were almost similar in both gestational age groups and, therefore, combined into one ventilation group (all ventilation). In the 28-d gestational age rabbits, the plasma CH50 activity of the nonventilated group was significantly higher than that of the 30-min (p < 0.05) and 60-min (p < 0.05) ventilated groups separately, and than that of the all ventilated group (p < 0.05). In the 29-d gestational age group, the plasma CH50 activity of the nonventilated rabbits was similar to that of the ventilated rabbits. In Figure 2, differences between the 28- and 29-d gestational age rabbits are presented.
The CH50 of the 28-d gestational age (open boxes) and 29-d gestational age (gray boxes) rabbits, that were not ventilated (no vent.) or ventilated for 30 min (30′ vent.) or 60 min (60′ vent.). Data of the 30- and 60-min ventilated rabbits in each gestational age group were not different and combined to one single ventilation group (all vent.). Data are presented as box graphs showing the median values (horizontal plane line), ranges of 50% around the median value (boxes), and the 10th and 90th percentile (error bars). ***p < 0.001 for 28-d compared with 29-d rabbits.
β- glucuronidase. In Table 3, differences between the ventilated and nonventilated rabbits in both gestational age groups are presented. In the 28-d gestational age group, theβ-glucuronidase concentration of the nonventilated rabbits was lower than that of the rabbits that were ventilated for 30 (NS) or 60 min (p< 0.05). The β-glucuronidase concentration of the all ventilated group was significantly higher (p < 0.05) than that of the nonventilated group. In the 29-d gestational age group, theβ-glucuronidase concentrations of the ventilated and nonventilated rabbits were not different.
The β-glucuronidase concentrations of the nonventilated rabbits in both the 28- and 29-d gestational age group were not different. However, the values of the 60-min ventilated and all ventilated rabbits in the 28-d gestational age group were significantly higher than the values for these rabbits in the 29-d gestational age group (p < 0.05 for the 60-min ventilated rabbits; p < 0.01 for all ventilated rabbits).
Total protein in plasma and alveolar wash. The total protein concentrations in plasma of the ventilated and nonventilated rabbits within each gestational age group and between the two gestational age groups were not different (data not shown). In Table 3, differences between the ventilated and nonventilated rabbits in both gestational age groups are presented. In the 28-d gestational age group, the total protein concentration in the alveolar wash of the nonventilated rabbits was significantly lower than that of the rabbits after 30 min (p < 0.05) and 60 min (p < 0.05) of artificial ventilation. The total protein concentration in the alveolar wash of the all ventilated group was significantly higher (p < 0.01) than that of the nonventilated group. In the 29-d gestational age group, the total protein concentration in the alveolar wash of the nonventilated rabbits did not differ significantly from that of the ventilated rabbits.
There was no difference between the total protein concentrations in the alveolar wash of the nonventilated rabbits in the 28- and 29-d gestational age groups. The 30-min ventilated rabbits and all ventilated rabbits of both gestational age groups differed significantly with regard to these concentrations (p < 0.05 for the 30-min ventilated rabbits;p < 0.01 for all ventilated rabbits).
Correlations. The total protein concentration of the alveolar wash of all rabbits was significantly correlated with the plasma concentration of fibrin monomers (ρ = 0.35, p = 0.019) andβ-glucuronidase (ρ = 0.37, p = 0.0146), and the plasma activity of CH50 (ρ = -0.30, p = 0.040) in these rabbits. These correlations were also significant in the 28-d gestational age group but not in the 29-d gestational age group (Table 4).
DISCUSSION
In this study, we have found that activation of clotting, complement, and circulating PMN occurs in plasma of preterm ventilated rabbits of 28-d gestational age with severe respiratory insufficiency. Simultaneously, we have observed a more pronounced protein leakage in the lungs of these rabbits than in the lungs of ventilated 29-d gestational age rabbits. In the 28-d gestational age rabbits, the concentration of protein in the airways was positively correlated with the concentration of fibrin monomers, andβ-glucuronidase in plasma and negatively correlated with the CH50 activity of plasma. The 30- or 60-min duration of ventilation did not result in significant differences within both gestational age groups with regard to leakage of protein in the lungs and activation of clotting, complement, and PMN in plasma.
Intravascular activation of the clotting system in the ventilated rabbits of the 28-d gestational age group was represented by higher plasma concentrations of fibrin monomers than in the nonventilated rabbits of that group. Intravascular and intraalveolar activation of clotting has been described in preterm newborn infants with severe RDS(19, 21, 34, 35) and likely contributes to respiratory insufficiency in these infants. Thrombin, the common product of extrinsic and intrinsic clotting, causes pulmonary endothelial injury directly and indirectly by activation of platelets and PMN, thus contributing to formation of pulmonary edema(36, 37). Intravascular fibrin thrombi, that have been demonstrated at autopsy in the lungs of preterm infants who had died of severe RDS, decrease surfactant synthesis due to impaired lung perfusion(34, 35). Furthermore, intraalveolar fibrin is a major component of hyaline membranes, which inactivate considerable amounts of surfactant(8).
The plasma CH50 activity was significantly reduced in the ventilated rabbits of the 28-d gestational age group. Low plasma CH50 activity indicates low plasma concentrations of one or more products of the classical complement pathway, which may be the result of insufficient synthesis(38), as has been described in preterm human newborns(39–41). The low CH50 activity in the ventilated 28-d gestational age rabbits, however, was not due to preexisting lower concentrations of complement products because of the higher CH50 activity in the nonventilated rabbits of that group. Therefore, this lower CH50 activity is likely caused by activation and subsequent consumption of complement components. Complement activation has been described in preterm and term newborn infants with severe respiratory distress. In these infants active components of the complement system such as C3a and C5a have been found in plasma(22, 42) and in tracheobronchial aspirates(23, 24). C3a and C5a are known to increase vascular permeability and might contribute to pulmonary edema formation(43). Furthermore, C5a stimulates chemotaxis, aggregation, and adherence of activated PMN to endothelial cells, especially in the lungs(44).
Active components of complement and clotting may have contributed to PMN activation and subsequent increased plasma β-glucuronidase concentrations in the ventilated rabbits of the 28-d gestational age group. An increase of the plasma β-glucuronidase concentration is considered to be a marker for release of bioactive agents by circulating activated PMN(45). Influx and accumulation of PMN into the lungs has been reported in infants with severe RDS(23–26). In humans, PMN release products such as elastase and leukotriene B4 and are thought to be involved in destruction of lung connective tissue and breakdown of pulmonary vascular endothelium(23–27).
We have found that total protein concentrations in the alveolar wash of ventilated rabbits were higher than that of nonventilated rabbits in both gestational age groups. The total protein concentration of fluid obtained by alveolar wash is representative for leakage of protein from the intravascular space into the small airways(3–5). Protein leakage into the small airways results in the formation of protein-rich pulmonary edema, which interferes with lung function(3–7, 9–12). In the ventilated rabbits of the 28-d gestational age group protein leakage in the small airways was significantly higher than in those of the 29-d gestational age group. Concomitantly, compliance and VEI were significantly lower, whereas peak inspiratory pressures used to ventilate the rabbits properly were significantly higher in the 28-d gestational age group than in the 29-d gestational age group. These findings are in agreement with Ikegamiet al.(28).
In the 28-d gestational age rabbits, the total protein concentration in the alveolar wash correlated significantly with the plasma concentration of fibrin monomers, β-glucuronidase, and the plasma activity of CH50. Activation of clotting, complement, and leukocytes may contribute to further lung injury in preterm animals and infants by intravascular and intraalveolar fibrin deposition and pulmonary edema formation according to the aforementioned mechanisms. Such activation process might explain, at least in part, the protein leakage that has been demonstrated in unventilated lung segments of preterm lambs(16).
We did not find differences between the 30- and 60-min ventilated rabbits within both gestational age groups regarding protein leakage in the lungs and clotting-complement-PMN activation in plasma. Considerable amounts of protein have been found in the small airways of preterm rabbits that were ventilated for 30 min(5, 28). Activation of complement and clotting has been demonstrated in preterm ventilated infants with RDS within 6 h of birth(21, 22). Although protein leakage in the lungs and protein activation in plasma occur early in the course of neonatal RDS, the 30-min difference between the ventilation subgroups, which was chosen arbitrarily in this study, might be too short to observe changes in these processes. Furthermore, one could argue that the absence of statistical significant differences between the 30- and 60-min ventilation groups can be caused by the rather small numbers of rabbits that have been studied.
The cause of the aforementioned activation of clotting, complement, and PMN is not yet clarified. Ventilator-mediated lung tissue injury can be accompanied by activation of factor XII and kallikrein(46), as has been demonstrated in preterm ventilated infants with severe RDS(20, 21). Activated factor XII contributes to clotting activation, whereas both activated factor XII and kallikrein contribute to complement activation(46, 47). In this study, activation of clotting, complement, and PMN occurred in plasma after onset of positive pressure ventilation in the 28-d gestational age rabbits. Concomitantly, the peak pressures used to ventilate these rabbits properly were higher than those used in the ventilated rabbits of the 29-d gestational age group. Because there was no spontaneous breathing control group surviving 30 or 60 min, the role of positive pressure ventilation in the development of protein leakage in the lungs and activation of clotting, complement, and PMN in plasma remains speculative. Our data do not show a relation between hypoxemia and acidosis at birth and activation of clotting, complement, and PMN, because we did not find signs of activation in the nonventilated rabbits of both gestational age groups. An association between perinatal asphyxia (hypoxemia and acidosis) and activation of clotting has been described in preterm ventilated infants with severe RDS(21). It could be argued that we have missed such association in the present study because blood samples were obtained from the nonventilated rabbits within a few minutes of birth. At the moment of blood sampling, duration of activation might not have been sufficient to achieve increased plasma concentrations.
We conclude that systemic activation of clotting, complement, and PMN is correlated with leakage of protein in the lungs of ventilated preterm rabbits of 28-d gestational age. This activation process is able to injure vascular endothelium in the lungs, which may contribute to protein-rich edema formation and intraalveolar fibrin deposition. However, it remains to be established whether a causal relationship between systemic activation of clotting, complement, and PMN on the one hand and pulmonary protein-rich edema formation on the other hand occurs in neonatal RDS. Further studies are required to elucidate the influence of positive pressure ventilation duration on leakage of protein in the lungs and activation of proteins and cells in plasma.
Abbreviations
- RDS:
-
respiratory distress syndrome
- PMN:
-
polymorphonuclear leukocytes
- CH50:
-
complement hemolytic activity of plasma
- VEI:
-
ventilator efficiency index
- PIP:
-
peak inspiratory pressure
References
Jefferies AL, Coates G, O'Brodovich H 1984 Pulmonary epithelial permeability in hyaline membrane disease. N Eng J Med 311: 1075–1080.
Bland RD, Carlton DP, Scheerer RG, Cummings JJ, Chapman DL 1989 Fluid balance in lambs before and after premature birth. J Clin Invest 84: 568–576.
Jobe A, Ikegami M, Jacobs H, Jones S, Conaway D 1983 Permeability of premature lamb lungs to protein and the effect of surfactant on that permeability. J Appl Physiol 55: 169–176.
Jobe A, Jacobs H, Ikegami M, Berry D 1985 Lung protein leaks in ventilated lambs: effect of gestational age. J Appl Physiol 58: 1246–1251.
Ikegami M, Berry D, El Kady T, Pettenazzo A, Seidner S, Jobe A 1987 Corticosteroids and surfactant change lung function and protein leaks in the lungs of ventilated premature rabbits. J Clin Invest 79: 1371–1378.
Ikegami M, Jobe AH, Tabor BL, Rider ED, Lewis JF 1992 Lung albumin recovery in surfactant-treated preterm ventilated lambs. Am Rev Respir Dis 145: 1005–1008.
O'Brodovich HM, Coates G 1988 Pulmonary edema in respiratory distress syndrome and bronchopulmonary dysplasia. In: Merritt TA, Northway WH, Boynton BR (eds) Bronchopulmonary Dysplasia, Blackwell Scientific Publications, Boston, 143–159.
Pinnas JL, Srunk RC, Fenton LJ 1979 Immunofluorescence in group B streptococcal infection and idiopathic respiratory distress syndrome. Pediatrics 63: 557–561.
Ikegami M, Jacobs H, Jobe A 1983 Surfactant function in the respiratory distress syndrome. J Pediatr 102: 443–447.
Ikegami M, Jobe A, Jacobs H, Lam R 1984 A protein from airways of premature lambs that inhibit surfactant function. J Appl Physiol 57: 1134–1142.
Fuchimukai T, Fujiwara T, Takahashi A, Enhorning 1987 Artificial pulmonary surfactant inhibited by proteins. J Appl Physiol 62: 429–437.
Ueda T, Ikegami M, Jobe A 1994 Surfactant subtypes.In vitro conversion, in vivo function, and effects of serum proteins. Am J Respir Crit Care Med 149: 1254–1259.
Scarpelli EM, Mautone AJ 1984 The surfactant system and pulmonary mechanics In: Robertson B, van Golde LMG, Batenburg JJ (eds) Pulmonary Surfactant. Elsevier Science Publishers, Amsterdam, pp 119–170.
Nilsson R, Grossmann G, Robertson B 1978 Lung surfactant and the pathogenesis of neonatal bronchiolar lesions induced by artificial ventilation. Pediatr Res 12: 249–255.
Nilsson R, Robertson B 1985 Bronchiolar epithelial lesions in spontaneously breathing premature newborn rabbits. Biol Neonate 48: 357–361.
Berry D, Jobe A, Ikegami M 1991 Leakage of macromolecules in ventilated and unventilated segments of preterm lamb lungs. J Appl Physiol 70: 423–429.
Roall JA, Levin DL 1987 Adult respiratory distress syndrome in pediatric patients. I. Clinical aspects, pathophysiology, and mechanisms of lung injury. J Pediatr 112: 169–180.
Sarnaik AP, Lieh-Lai M 1994 Adult respiratory distress syndrome in children. Pediatr Clin N Am 41: 337–364.
Schmidt B, Vegh P, Weitz J, Johnston M, Caco C, Roberts R 1992 Thrombin/antithrombin III complex formation in the neonatal respiratory distress syndrome. Am Rev Respir Dis 145: 767–770.
Saugstad OD, Buo L, Johansen HT, Roise O, Aasen AO 1992 Activation of the plasma kallikrein-kinin system in respiratory distress syndrome. Pediatr Res 32: 431–435.
Brus F, van Oeveren W, Okken A, Bambang Oetomo S 1994 Activation of the plasma clotting, fibrinolytic and kinin-kallikrein system in preterm infants with severe idiopathic respiratory distress syndrome. Pediatr Res 36: 647–653.
Schrod L, Frauendienst-Egger G, Stockhausen von HB, Kirschfink M 1992 Complement fragment C3a in plasma of asphyxiated neonates. Eur J Pediatr 151: 688–692.
Groneck P, Götze-Speer B, Oppermann M, Eiffert H, Speer CP 1994 Association of pulmonary inflammation and increased microvascular permeability during the development of bronchopulmonary dysplasia: a sequential analysis of inflammatory mediators in respiratory fluids of high-risk preterm neonates. Pediatrics 93: 712–718.
Groneck P, Reuss D, Götze-Speer B, Speer CP 1993 Effects of dexamethasone on chemotactic activity and inflammatory mediators in tracheobronchial aspirate of preterm infants at risk for chronic lung disease. J Pediatr 122: 938–944.
Ogden BE, Murphy SA, Saunders GC, Pathak D, Johnson JD 1984 Neonatal lung neutrophils and elastase/proteinase inhibitor imbalance. Am Rev Respir Dis 130: 817–821.
Merritt TA, Cochrane CG, Holcomb K, Bohl B, Hallman M, Strayer D, Edwards DK 1983 Elastase and α-1-proteinase inhibitor activity in tracheal aspirates during respiratory distress syndrome. J Clin Invest 72: 656–666.
Speer CP, Ruess D, Harms K, Herting E, Gefeller O 1993 Neutrophil elastase and acute pulmonary damage in neonates with severe respiratory distress syndrome. Pediatrics 91: 794–799.
Ikegami M, Jobe AH, Seidner S, Yamada T 1989 Gestational effects of corticosteroids and surfactant in ventilated rabbits. Pediatr Res 25: 32–37.
Plötz FB, van Oeveren W, Aloe L, Riley M, Hultquist KA, Bartlett RH, Wildevuur Ch RH 1991 Prophylactic administration of tranexamic acid preserves platelet numbers during extracorporeal circulation in rabbits. ASAIO Trans 37: 416–417.
Mayer MM 1971 Complement and complement fixation. In: Kabat EA (ed) Experimental Immunochemistry Charles C Thomas, Springfield, IL, pp 133–140.
Baehner RL 1975 Subcellular distribution of nitroblue tetrazolium reductase (NBT-R) in human polymorphonuclear leukocytes (PMN). J Lab Clin Med 86: 785–792.
Lowry OH, Rosenbrough NJ, Farr AL, Randall RJ 1951 Protein measurement with the Folin phenol reagent. J Biol Chem 193: 265–275.
Notter RH, Egan EA, Kwong MS, Holm BA, Shapiro DL 1985 Lung surfactant replacement in premature lambs with extracted lipid from bovine lung lavage: effects of dose, dispersion technique and gestational age. Pediatr Res 19: 569–577.
Stark CR, Abramson D, Erkan V 1968 Intravascular coagulation and hyaline membrane disease of the newborn. Lancet 2: 1180–1181.
Peters M, Ten Cate JW, Breederveld C, De Leeuw R, Emeis J, Koppe J 1984 Low antithrombin III levels in neonates with idiopathic respiratory distress syndrome: poor prognosis. Pediatr Res 18: 273–276.
Cooper JA, Feustel PJ, Line BR, Malik AB 1986 Pulmonary epithelial clearance of 99 mTc-DTPA after thrombin-induced pulmonary microembolism. Am Rev Respir Dis 134: 734–738.
Malik AB, Horgan MJ 1987 Mechanisms of thrombin-induced lung vascular injury and edema. Am Rev Respir Dis 136: 467–470.
Adinolfi M 1977 Human complement. Onset and site of synthesis during fetal life. Am J Dis Child 131: 1015–1023.
Strunk RC, Fenton LJ, Gaines JA 1979 Alternative pathway of complement activation in full term and premature infants. Pediatr Res 13: 641–643.
Notarangelo LD, Chirico G, Chiara A, Colombo A, Rondini G, Plebani A, Martini A, Ugazio AC 1984 Activity of classical and alternative pathways of complement in preterm and small for gestational age infants. Pediatr Res 18: 281–285.
Berger M 1990 Complement deficiency and neutrophil dysfunction as risk factors for bacterial infection in newborns and the role of granulocyte transfusion in therapy. Rev Infect Dis 12:S401–S409.
Plötz FB, van Oeveren W, Bartlett RH, Wildevuur Ch RH 1993 Blood activation during neonatal extracorporeal life support (ECLS). J Thorac Cardiovasc Surg 105: 823–832.
Weiland JE, Davis WB, Holter JF, Mahammed JR, Dorinsky PM, Gadek JE 1986 Lung neutrophils in the adult respiratory distress syndrome. Clinical and pathophysiologic significance. Am Rev Respir Dis 133: 218–225.
Goldstein IM 1988 Complement: biologically active products. In: Gallin JI, Goldstein IM, Snyderman R (eds) Inflammation: Basic Principles and Clinical Correlates. Raven Press, New York, pp 55–67.
Goldstein JM, Hoffstein ST, Weissmann G 1975 Influence of divalent cations upon complement-mediated enzyme release from human polymorphonuclear leukocytes. J Immunol 115: 665–670.
Kaplan AP, Silverberg M 1987 The coagulation-kinin pathway of human plasma. Blood 70: 1–15.
Hathaway WE 1992 Normal hemostatic mechanisms. In: Polin RA, Fox WW (eds) Fetal and Neonatal Physiology, Vol 2. WB Saunders, Philadelphia, pp 1365–1368.
Acknowledgements
The authors thank Peter Dijk and Frans Plötz for assistance during the animal experiments, Johan Haan for technical assistance, and Annalie van de Vijver and Miep Helfrich for critically reading the manuscript.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Brus, F., Van Oeveren, W., Heikamp, A. et al. Leakage of Protein into Lungs of Preterm Ventilated Rabbits Is Correlated with Activation of Clotting, Complement, and Polymorphonuclear Leukocytes in Plasma. Pediatr Res 39, 958–965 (1996). https://doi.org/10.1203/00006450-199606000-00006
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/00006450-199606000-00006
This article is cited by
-
Activatie van plasma-eiwitten en bloedcellen bij het neonataal respiratoir distress-syndroom: pathogenetische en therapeutische aspecten
Tijdschrift voor kindergeneeskunde (2000)