Abstract
The role of vitamin D in ameloblasts and odontoblasts has been studied experimentally in rodents. Dental dysplasias have also been reported in clinical studies of children with rickets. Vitamin D acts via a nuclear receptor which binds the major metabolite, 1,25-dihydroxyvitamin D3, and positively or negatively controls the expression of specific genes. The most extensively studied markers of 1,25-dihydroxyvitamin D3 action are calbindin-D9k, calbindin-D28k, and osteocalcin. Therefore, to study in more detail the potential role of 1,25-dihydroxyvitamin D3 in human dental development, 1,25-dihydroxyvitamin D3 receptor (VDR) was localized by immunofluorescence in forming teeth (8-26 wk of gestation). Calbindin-D28k was also mapped by immunoperoxidase in antenatal and postnatal forming and formed teeth. VDR were detected in both dental epithelium and mesenchyme of bud, cap, and bell stages of tooth germs. Nuclei of overtly differentiated ameloblasts and odontoblasts were also immunostained. Calbindin-D28k was present in differentiated ameloblasts and odontoblasts. The presence of VDR and calbindin-D28k in ameloblasts and odontoblasts suggests that 1,25-dihydroxyvitamin D3 may contribute to the regulation of enamel and dentin formation, as classically reported for bone formation. Finally, the early appearance of VDR supports the concept that 1,25-dihydroxyvitamin D3 may also control forward stages of tooth crown development in humans.
Similar content being viewed by others
Main
Vitamin D deficiency appears during pregnancy with a relatively high prevalence, especially when the last trimester occurs during winter(1, 2). The resulting maternofetal vitamin D deficiency has been associated with neonatal hypocalcemia(3, 4) and a delay in neonatal growth, and it may also induce disturbances in enamel formation(4). However, limited data have been obtained on the molecular basis of vitamin D action in the early stages of human development(5).
The intracellular action of 1,25(OH)2D3, the active form of vitamin D, is initiated by binding of the hormone to specific VDR in nuclei of target cells. The 1,25(OH)2D3-VDR complex binds as homodimers or heterodimers with other nuclear receptors(6). These complexes bind to target genes and regulate gene expression in a tissue and stage-specific manner(7). These vitamin D-dependent molecules mediate the biologic actions of the hormone. Among the various vitamin D-responsive genes, calbindin-D28k is expressed in teeth(8). Kim et al.(9) identified target cells for 1,25(OH)2D3 in developing rodent teeth by autoradiography. Immunoreactivity for VDR(8) is present in all progenitor cells and in differentiated ameloblasts and odontoblasts. At this latter stage of development, the expression of VDR, calbindin-D9k, and calbindin-D28k is jointly up-regulated by 1,25(OH)2D3(18). Therefore, ameloblasts and odontoblasts, as well as progenitor dental cells, have been described to be target cells for 1,25 (OH)2D3 in rodents(8).
Calbindin-D28k has been demonstrated in mature odontoblasts of human formed teeth(10), whereas VDR has been described only in central pulp cells(11). Immunocytochemical(12) and in situ hybridization studies(13) in developing human fetal teeth have demonstrated the presence of another vitamin D-dependent [for review, see Lowe et al.(14)] matrix protein: osteonectin. However, the expression of potential vitamin D-responsive proteins in human ameloblasts has not been investigated. In the present study, the temporospatial appearance of VDR and a vitamin D-dependent calcium-binding protein (calbindin-D28k) was investigated by immunocytochemistry during human odontogenesis.
METHODS
Immunolocalization in human teeth was performed as follows.
Tissue collection and preparation. Tooth germs (n = 50) from 15 human embryos and fetuses were obtained from legally approved medical abortions induced by prostaglandins, spontaneous abortions, and newborn infants. Informed consent was obtained according to the guidelines of the Declaration of Helsinki. The fetuses studied ranged from 8 to 26 wk of gestation. Embryonic stage and fetal age were determined by anthropometric measurements and/or histologic maturation. For VDR immunodetection in tooth germ, 10 mandibles were frozen immediately after delivery, plunged into liquid nitrogen, and stored frozen at -80°C. After embedding in Tissue-Teck O.C.T Coump (Miles Scientific, Elkhart, IN), serial 7-μm sections were cut sagittally with a cryostat at -20°C, transferred onto poly-L-lysine (Sigma Chemical Co., la Verpillère, France) coated glass slides, and stored at-20°C. For calbindin-D28k immunodetection in the forming tooth, five mandibles were fixed in 10% formalin for several days at 4°C, decalcified or not, dehydrated, and embedded in paraffin. Furthermore, the forming postnatal tooth germs (n = 10) were obtained with the parent's consent either from deceased children during autopsy for diagnosis and/or scientific purposes or from surgical hemimandibulectomy. Sections (5 μm) were cut, deparaffinized, and rehydrated. For calbindin-D28k in the formed tooth, 10 premolars extracted for orthodontic reasons were collected and immediately immersed in 4% buffered phosphonoformatic acid. Dental pulps were microdissected under a microscope, dehydrated, and embedded in paraffin.
VDR fluorescence immunolocalization. To saturate nonspecific binding sites, each section was treated with normal goat serum (Sigma Chemical Co.) for 30 min, diluted to 1:30 in PBS (0.1 mol/L, Eurobio) at room temperature. They were then incubated with monoclonal rat anti-VDR (Chemicon, Temecula, CA) diluted to 1:20, 1:50, and 1:100 in PBS. Sections were maintained overnight at 4°C in a humid atmosphere. For control staining, VDR antibody was replaced by rat IgG (Nordic, Tilburg, The Netherlands) with the same dilutions. After rinsing in PBS, sections were incubated with biotinylated anti-rat IgG antibodies (Sigma Chemical Co.) diluted to 1:50 for 1 h at 37°C. They were then rinsed in PBS (0.1 mol/L) and incubated with streptavidin-FITC complex (FITC, Sigma Chemical Co.) for 30 min (dilution 1:200) at 37°C. Sections were then rinsed in PBS containing 2% BSA and maintained in PBS with 2% BSA for 2 h in an agitator. Finally, sections were mounted in hydrophilic fluorescence medium (Biosys, Compiègne, France) and examined under epifluorescence with an Axioplan light microscope(Zeiss).
Calbindin-D28K immunolocalization. Antibodies were raised against rat kidney calbindin-D28k in rabbits(15). Their cross-reactivity with human calbindin-D28k has been classically established(16). All rinsings and incubations were performed in 0.05 mol/L Tris-HCl at pH 7.3 (Tris). Endogenous peroxidases were blocked by a 30-min treatment with 3% H2O2 in distilled water. Sections were then rinsed and incubated for 30 min with normal goat serum diluted to 1:10 with 1% BSA. They were then incubated for 1 h at 37°C with 1:1000 rabbit polyclonal anti-calbindin-D28k antibodies diluted in Tris-1% normal goat serum, and then rinsed. The sections were then incubated for 30 min with 1:100 peroxidase secondary antibodies (anti-rabbit IgG; Sigma Chemical Co.). The sections were rinsed and incubated with rabbit anti-peroxidase complex (Sigma Chemical Co.) at a dilution of 1:100 for 30 min at 37°C. The sections were finally rinsed with Tris. Immunoreactive sites were visualized with 3,3′-diaminobenzidine (Sigma Chemical Co.; 5 mg/10 mL) in 0.005 mol/L Tris-HCl, pH 7.6, with 0.03% H2O2 for 10 min. Sections were rinsed in water, stained with Harris hematoxylin, dehydrated, mounted in DePex(BDH Laboratory, Poole, UK), and examined with an Orthoplan light microscope(Zeiss).
RESULTS
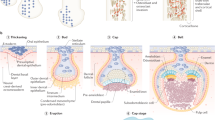
VDR immunolabeling during early stages of tooth formation (Fig. 1). Bud, cap, and bell stages in human mandibles from 8 to 26 wk were examined (Fig. 1, A-F). Immunolabeling for VDR was detected in the dental epithelium throughout morphogenesis and histodifferentiation (Fig. 1, A, bud stage, and B, cap stage). Buccal epithelium (Fig. 1C) and mandibular bone (Fig. 1D) were used as positive controls for VDR immunoreactivity. Negative controls (Fig. 1F, cap stage) with nonspecific IgGs provided low background levels. Morphologic staining illustrates the detailed appearance of the cap stage epithelium, especially enamel knot (Fig. 1E), as VDR staining was very marked in this area (Fig. 1B).
(A) VDR immunolabeling in bud stage tooth germ (second primary molar of a 10-wk-old human fetus; ×400, antibody 1:50). The staining appears present mainly in the epithelial cells.N, Nucleus; C, cytoplasm. (B) VDR immunolabeling in cap stage tooth germ (first primary molar of a 16-wk-old human fetus;×200, antibody 1:50). The staining is distributed mainly in the epithelium, but adjoining ectomesenchymal cells are also immunolabeled.IDE, Inner dental epithelium; ODE, outer dental epithelium; EK, enamel knot. (C) VDR immunolabeling in the mandibular bone of a 26-wk-old human fetus (×200, antibody 1:50). The first control tissue, forming bone, contains immunopositive osteoblastic cells at several stages of differentiation and maturation. OP, Osteoprogenitor cells; OB, osteoblasts; OC, osteocytes;MO, osteoid matrix. (D) VDR immunolabeling in buccal epithelium of a 26-wk-old human fetus (×400, antibody 1:50). The second control tissue, buccal epithelium, appears to show stained cells, with characteristic subcellular distribution of immunoreactive patches inside the nuclei (white arrows). N, Nucleus; E, epithelium; B, basal membrane; C, connective tissue.(E) Section of cap stage tooth germ (second primary molar of an 11-wk-old human fetus; ×200) which illustrates the morphology corresponding to that in B. DL, Dental lamina; EK, enamel knot; DM, dental ectomesenchyme; IDE, inner dental epithelium; ODE, outer dental epithelium. (F) Immunocytochemical control in cap stage tooth germ (second primary molar of an 11-wk-old human fetus; ×200). The background level obtained when the primary antibodies are omitted appears low. DL, Dental lamina;EO, enamel organ; DM, dental ectomesenchyme.
VDR and Calbindin-D28k immunolabeling during differentiation of ameloblasts and odontoblasts (Fig. 2). In the bell stage tooth germ, the terminal differentiation of ameloblasts and odontoblasts may be followed from the cervical loop to the lateral sides (Fig. 2A), as far as the tip (Fig. 2B) of the cusp. VDR were detected in the dental epithelium (Fig. 2A), even before complete ameloblast differentiation. In the dental mesenchyme, immunoreactive VDR were also present in morphologically undifferentiated cells (Fig. 2A) and in fully polarized odontoblasts (Fig. 2B). VDR labeling was mainly nuclear with, however, some cytoplasmic immunoreactivity (Fig. 2, A, B,and E). VDR was also distributed in distinct patches inside the nucleus (Fig. 2, A and E). Calbindin-D28k was immunostained in bell stage preameloblasts in undecalcified forming tooth (Fig. 2C) and was present in the cytoplasm of polarized odontoblasts (Fig. 2D) in undecalcified formed teeth.
VDR and calbindin-D28k immunolabeling in differentiating and differentiated ameloblasts and odontoblasts. (A) VDR immunolabeling in bell stage of a second primary molar in a 26-wk-old human fetus: lateral aspect of the cusp (×400, antibody 1:50). Nuclear labeling appears condensed in defined areas (small arrows).IDE, Inner dental epithelium containing preameloblasts;DM, dental ectomesenchyme. (B) VDR immunolabeling in bell stage of a second primary molar in a 26-wk-old human fetus: dental papilla located at the cusp tip (×400, antibody 1:50). Odontoblasts as well as other cells of the dental ectomesenchyme contain immunoreactivity for VDR.O, Odontoblasts; DP, adjacent dental papilla.(C) Calbindin-D28k immunolabeling in bell stage tooth germ (first primary molar of a 16-wk-old human fetus; ×400, antibody 1:1000). Calbindin-D28k is present during the differentiation of ameloblastic cells but not detected in the ectomesenchyme. In odontoblasts, the immunonegativity shown on decalcified samples must be interpreted carefully, as decalcifying agents may artefactually extract calbindin-D from the tissues(17). PA, Preameloblasts; DM, dental mesenchyme. (D) Calbindin-D28k immunolabeling in mature dental pulp(premolar of a 12-y-old child). Only odontoblasts are labeled in formed dental pulp. O, Odontoblasts; DP, dental pulp. (E) VDR immunolabeling in bell stage of a second primary molar in a 26-wk-old human fetus: enamel organ located at the cusp tip (×400, antibody 1:50). Similar to that seen in B, nuclear labeling inside ameloblasts appears condensed in defined areas (small arrows). A, Ameloblasts; E, enamel.
Calbindin-D28k in differentiated ameloblasts (Fig. 3). The presence of calbindin-D28k was related to the differentiation of ameloblasts. No immunoreactivity was present in dental lamina (Fig. 3A), in bud and cap stages (not shown), at which ameloblasts and odontoblasts were still not differentiated. In developing bell-staged teeth, the cervical loop area (Fig. 3B) contained immunonegative cells. In contrast, ameloblasts appeared to contain calbindin-D28k at the secretion stage (Fig. 3C). The immunoreactivity was evenly distributed in all ameloblasts (Fig. 3C). Immunoreactive controls were negative (Fig. 3D). The immunoreactivity of ameloblasts at the maturation stage was alternatively positive and negative (Fig. 3E). All cells were negative in the reduced enamel organ (Fig. 3F). Odontoblasts were immunonegative in these decalcified samples.
Distribution of calbindin-D28k immunostaining in tooth germs of a 1-mo-old infant. (A) Calbindin-D28k immunostaining in the dental lamina of a first premolar (×400, antibody 1:1000). No immunoreactive cells are observed. DL, Dental lamina. (B) Calbindin-D28k immunostaining in the cervical loop of a permanent incisor germ(×400, antibody 1:1000). Similar to dental lamina, all tissues are immunonegative. IDE, Inner dental epithelium; ODE, outer dental epithelium; DM, dental ectomesenchyme. (C) Calbindin-D28k immunostaining in the same tooth (×400, antibody 1:1000). Immunolabeling is restricted to ameloblasts. A, Secretion stage ameloblasts. (D) Control staining in a serial section vs C(×400). Low background is present throughout the section. A, Ameloblasts. (E) Calbindin-D28k immunostaining in the same tooth(×400, antibody 1:1000). A, Irregular immunolabeling in maturation stage ameloblasts. Solid arrowheads correspond to maximal staining, empty arrowheads correspond to minimal staining.(F) Calbindin-D28k immunostaining in the same tooth (×400, antibody 1:1000). Immunolabeling is negligible. A, Ameloblasts in reduced enamel organ.
DISCUSSION
VDR distribution in human forming teeth. Tooth morphogenesis results from invagination of the epithelium inside the ectomesenchyme of the first branchial arch(17, 18). This process and the subsequent histodifferentiation and terminal cytodifferentiation of ameloblasts and odontoblasts are secondary to epithelio-mesenchymal interactions(19). When overtly differentiated, polarized cells elaborate mineralized tissues, i.e. enamel for ameloblasts and dentin for odontoblasts(20). These processes result from a coordinated action of growth factors on progenitor and differentiated cells(17–20). Although numerous in vivo and in vitro investigations have been devoted to rodents, the identification of target cells for growth factors and hormones has been less extensively studied in human teeth. The receptors for various growth factors such as nerve growth factor receptors, p75NGF, and trkA(21, 22), and epithelial growth factor receptor(23–26) have been identified during human dental development. VDR have been previously shown in human teeth, but only in fully formed teeth(11).
The primary objective of the present study was therefore to immunolocalize VDR from early developmental stages until the process of mineralization in human forming teeth. To validate the immunochemical methodology and in agreement with previous observations(27–29), the presence of VDR was investigated in epithelium and forming bone. VDR has been detected throughout the differentiation process of forming bone, in progenitor cells, as well as osteoblasts and osteocytes. Moreover, the subcellular pattern of VDR (diffuse in the cytoplasm and distributed in distinct patches in the nucleus) was obtained in all immunopositive cell types and closely corresponded to the previously described VDR distribution(11).
Using these immunofluorescent techniques, the present study showed that VDR may also be expressed in early stages of tooth morphogenesis, in contrast to previous studies in which VDR was studied only after the bell stage had already been reached(8, 9, 11, 30). Epithelium and mesenchyme were immunoreactive for VDR in bud, cap, and bell stages of tooth germs. Consequently, vitamin D may control these early stages of odontogenesis. Morphogenesis and the differentiation pattern of ameloblasts and odontoblasts are both affected in vitamin D-deficient rats(31). At the present time, numerous vitamin D-dependent molecules in other tissues [for review, see Berdal et al.(32)] have also been suggested to contribute to epithelio-mesenchymal interactions leading to tooth morphogenesis and cell differentiation(19, 20). These molecules may therefore be potentially involved in the control of development by vitamin D, as may be the case for matrix proteins [fibronectin(33) and collagen type I(34)], growth factors [(nerve growth factor(35) and transforming growth factor-β(36)] and their receptors [epidermal growth factor receptor(37)], and transcription factors [Msx-2(38)].
VDR and Calbindin-D28k in human ameloblasts and odontoblasts. Defects in dental mineralization observed in dietary vitamin D deficiency or vitamin D-resistant rickets are classically described(39, 40). They were essentially considered to be related to calcium and phosphorus imbalances-hypocalcemia leading to enamel hypoplasia and hypophosphatemia leading to interglobular dentin formation(41). Furthermore, VDR were detected in human teeth essentially in cells not involved in the elaboration of dental tissues(11). In contrast, the present study supports that VDR are present in ameloblasts and odontoblasts of human forming teeth, as shown in rats(8). Vitamin D could therefore play a role in the control of the formation of mineralized tissues by acting on these cells. After their terminal differentiation, ameloblasts and odontoblasts secrete an extracellular matrix which ultimately undergoes mineralization(42). This process results from a coordinated expression of matrix proteins and molecules involved in calcium handling (calcium pump, calbindin-D9k, and calbindin-D28k). Most of these molecules are commonly synthesized under the control of 1,25(OH)2D3 in the tissues involved in calcium homeostasis: bone, intestine, or kidney(14). A vitamin D-dependent expression of calbindin-D(8) has been specifically demonstrated in rat ameloblasts and odontoblasts(43). The second objective of this study was therefore to investigate the developmental pattern of expression of one of these proteins. Calbindin-D28k was immunolocalized with the antibodies used previously in rat teeth(44–46), because they are cross-reactive with human calbindin-D28k(16). The present data in human samples closely correspond to the immunolabeling observed in rodents(10, 32, 44–48), especially in ameloblasts during the presecretion, secretion, and maturation stages of amelogenesis. The observed variations support the notion that calcium transport and homeostasis are finely tuned throughout the two steps of enamel mineralization, as previously proposed(44).
In conclusion, the present study supports the notion that vitamin D may control human odontogenesis from morphogenesis up until complete mineralization. The control of vitamin D synthesis and/or supply may therefore be critical for tooth development during pregnancy and in young children. This factor should be considered additional to the general nutritional status of the mother and child, as its imbalance leads to dental dysplasia but also to a secondary increased susceptibility to dental decay(49).
Abbreviations
- 1,25(OH)2D3:
-
1,25-dihydroxyvitamin D3
- VDR:
-
vitamin D receptor
References
Garabédian M, Ben-Mekhbi H 1991 Is vitamin D-deficiency rickets a public health problem in France and Algeria? In: Glorieux FH (ed) Rickets Nestlé Ltd, Vevey/Raven Press, New York, pp 215–221.
Zeghoud F, Thoulon JM, Gillet JY, Chabert P, Garabédian M 1991 Effects on sunshine on vitamin D status of pregnant women in France. J Gynecol Obstet Biol Reprod 20: 685–690.
Mallet E, Henocq A, De Ménibus CH 1991 Effects of vitamin D supplementation in pregnant women on the frequency of neonatal hypocalcemia In: Norman AW, Bouillon R, Thomasset M (eds) Vitamin D: A Pluripotent Steroid Hormone. Gruyter, New York, pp 869–870.
Cockburn F, Belton NR, Purvis RJ, Giles MM, Brown JK, Turner TL, Wilkinson EM, Forfar JO, Barrie WJ, McKay GS, Pocock SJ 1980 Maternal vitamin D intake and mineral metabolism in mothers and their newborn infants. BMJ 281: 11–14.
Brun P, Dupret JM, Perret C, Thomasset M, Mathieu H 1987 Vitamin D-dependent calcium-binding proteins (CaBPs) in human fetuses: comparative distribution of 9K CaBP and 28K CaBP mRNAs during development. Pediatr Res 21: 362–367.
Cheskis B, Freedman LP 1994 Ligand modulates the conversion of DNA-bound vitamin D3 receptor (VDR homodimers into VDR-retinoid X receptor heterodimers). Mol Cell Biol 14: 3329–3338.
Malloy P, Hughes M, Pike JW, Feldman D 1991 Vitamin D receptors mutation and hereditary 1,25-dihydroxyvitamin D resistant rickets. In: Norman AW, Bouillon R, Thomasset M (eds) Vitamin D-Gene Regulation. Gruyter, New York, 116–124.
Berdal A, Hotton D, Pike JW, Mathieu H, Dupret JM 1993 Cell- and stage-specific expression of vitamin D receptor and calbindin-D genes in rat incisor. Regulation by 1,25-dihydroxyvitamin D3. Dev Biol 155: 172–179.
Kim YS, Clark SA, Stumpf WE, DeLuca HF 1985 Nuclear uptake of 1,25-dihydroxyvitamin D3 in developing rodent teeth: an autoradiographic study. Anat Rec 212: 301–306.
Magloire H, Joffre A, Azerad J, Lawson DEM 1988 Localization of 28 kD calbindin in human odontoblasts. Cell Tissue Res 254: 341–348.
Clark SA, Dame MC, Kim YS, Stumpf WE, DeLuca HF 1985 1,25- Dihydroxyvitamin D3 in teeth or rats and humans: receptors and nuclear localization. Anat Rec 212: 250–254.
Reichert T, Storkel S, Beker K, Fisher LW 1992 The role of osteonectin in human tooth development: an immunohistological study. Calcif Tissue Int 50: 468–472.
Mundlos S, Schwahn B, Reichert T, Zabel B 1992 Distribution of osteonectin mRNA and protein during human embryonic and fetal development. J Histochem Cytochem 40: 283:29291
Lowe KE, Maiyar AC, Norman AW 1992 Vitamin D-mediated gene expression. CR Euk Gene Expr 2: 65–109.
Intrator S, Elion J, Thomasset M, Brehier A 1985 Purification, immunological and biochemical characterization of rat 28kD cholecalcin (cholecalciferol-induced calcium binding protein). Biochem J 231: 89–95.
Hirsch EC, Mouatta A, Thomasset M, Javoy-Agid G, Agid Y, Graybiel AM 1992 Expression of calbindin D-28k-like immunoreactivity in catecholaminergic cell group of human midbrain: normal distribution and distribution in Parkinson's disease. Neurodegeneration 1: 83–93.
Ruch JV 1987 Determinisms of odontogenesis. Rev Biol Celular 14: 1–112.
Slavkin HC 1988 Gene regulation in the development in oral tissues. J Dent Res 67: 1142–1149.
Thesleff I, Partanen AM, Vainio S 1991 Epithelial-mesenchymal tissue interactions in tooth morphogenesis: the roles of extracellular matrix, growth factors, and cell surfaces receptors. J Craniofac Genet Dev Biol 11: 229–237.
Slavkin HC 1991 Molecular determinants during dental morphogenesis and cytodifferentiation: a review. J Craniofac Genet Dev Biol 11: 338–339.
Mitsiadis TA, Couble P, Dicou E, Rudkin BB, Magloire H 1993 Patterns of nerve growth factor, proNGF, and p75 NGF receptor expression in the rat incisor: comparison with expression in the molar. Differentiation 54: 161–175.
Christensen LR, Mollgard K, Kjaer I, Janas MS 1993 Immunocytochemical demonstration of nerve growth factor receptor (NGF-R) in developing human fetal teeth. Anat Embryol 188: 247–255.
Partanen AM, Thesleff I 1987 Localization and quantification of I125-epidermal growth factor binding in mouse embryonic tooth and other embryonic tissues at different developmental stages. Dev Biol 120: 186–197.
Cam Y, Neumann MR, Ruch JV 1990 Immunolocalization of transforming growth factor 1 and epidermal growth factor receptor epitopes in mouse incisors and molars with a demonstration of in vitro production of transforming activity. Arch Oral Biol 35: 813–822.
Heikinheimo K, Voutilainen R, Happonen RP, Miettinen PJ 1993 EGF receptor and its ligands, EGF and TGF-, in developing and neoplastic human odontogenic tissues. Int J Dev Biol 37: 387–396.
Davideau JL, Sahlberg C, Thesleff I, Berdal A 1995 EGF receptor expression in mineralized tissues: an in situ hybridization and immunocytochemical investigation in rat and human mandibles. Connect Tissue Res 32: 47–53.
Clemens TL, Garret KP, Zhou X, Pike JW, Haussler MR, Dempster DW 1988 Immunocytochemical localization of the 1,25-dihydroxyvitamin D3 receptor in target cells. Endocrinology 122: 1224–1230.
Boivin G, Mesguich P, Pike JW, Bouillon R, Meunier PJ, Haussler MR, Dubois PM, Morel G 1987 Ultrastructural immunocytochemical localization of endogenous 1,25-dihydroxyvitamin D3 and its receptors in osteoblasts and osteocytes from neonatal mouse and rat calvaria. Bone Miner 3: 125–136.
Balmain N, Hauchecorne M, Pike WJ, Cuisinier-Gleizes P, Mathieu H 1993 Distribution and subcellular immunolocalization of 1,25-dihydroxyvitamin D3 receptors in rat epiphyseal cartilage. Cell Mol Biol 39: 339–350.
Kim YS, Stumpf WE, Clark SA, Sar M, DeLuca HF 1983 Target cells for 1,25-dihydroxyvitamin D3 in developing rat incisor teeth. J Dent Res 62: 58–59.
Berdal A, Balmain N, Cuisinier-Gleizes P, Mathieu H 1987 Histology and microradiography of early post-natal molar tooth development in vitamin-D deficient rats. Arch Oral Biol 32: 493–498.
Berdal A, Hotton D, Kamyab S, Cuisinier-Gleizes P, Mathieu H 1991 Subcellular co-localization and co-variations of two vitamin D-dependent calcium-binding proteins in rat ameloblasts. Arch Oral Biol 36: 715–725.
Lesot H 1986 Cell-Matrix interactions during odontoblast differentiation. In: Robert L (ed) Front Matrix Biology. S Karger, Basel, 139–159.
Lichtler A, Stower ML, Angilly J, Kream B, Rowe DW 1989 Isolation and characterization of the rat alpha 1 (I) collagen promoter. Regulation by 1,25-dihydroxyvitamin D3. J Biol Chem 264: 3072–3077.
Wion D, Mac Grogan D, Neveu I, Jehan F, Houlgatte R, Brachet P 1991 1,25-dihydroxyvitamin D3 is a potent inducer of nerve growth factors synthesis. J Neurosci Res 28: 110–114.
Bègue-Kirn C, Smith AJ, Ruch JV, Wozney JM, Purchio A, Hartmann D, Lesot H 1992 Effects of dentin proteins, transforming growth factor β1 and bone morphogenetic protein 2 on the differentiation of odontoblasts in vitro. Int J Dev Biol 36: 491–503.
Desprez PY, Poujol D, Falette N, Lefebvre MF, Saez S 1991 1,25-Dihydroxyvitamin D3 increases epidermal growth factor receptor gene expression in BT-20 breast carcinoma cells. Biochem Biophys Res Commun 176: 1–6.
Hodgkinson JE, Davidson CL, Beresford J, Sharpe PT 1993 Expression of a human homeobox-containing gene is regulated by 1,25(OH)2D3 in bone cells. Biochim Biophys Acta 1174: 11–16.
Large DM, Mawer EB, Davies M 1984 Case report: dystrophic calcification, cataracts and enamel hypoplasia due to long-standing privational vitamin D deficiency. Bone 5: 215–218.
Limeback H, Schlumbhom C, Sen A, Nikiforuk G 1992 The effects of hypocalcemia/hypophosphatemia on porcine bone and dental hard tissues in an inherited form of type 1 pseudo-vitamin D deficiency rickets. J Dent Res 71: 346–352.
Nikiforuk G, Fraser D 1981 The etiology of enamel hypoplasia: a unifying concept. J Pediatr 98: 888–893.
Berdal A, Papagerakis P, Hotton D, Bailleul-Forestier I, Davideau JL 1995 Ameloblasts and odontoblasts, target-cells for 1,25-dihydroxyvitamin D3: a review. Int J Dev Biol 39: 257–262.
Hotton D, Davideau JL, Bernaudin JF, Berdal A 1995 In situ hybridization of calbindin-D28k transcripts in undecalcified sections of the rat continuously erupting incisor. Connect Tissue Res 32: 137–143.
Berdal A, Nanci A, Balmain N, Thomasset M, Bréhier A, Cuisinier-Gleizes P, Mathieu H 1989 Immunolocalization and radioimmunoassay of calbindins during amelogenesis in normal and vitamin-D deficient rats. In: RW Fearnhead (eds) Tooth Enamel V. Florence Publishers, Yokohama, 154–158.
Berdal A, Nanci A, Smith CE, Ahluwalia JP, Thomasset M, Cuisinier-Gleizes P, Mathieu H 1991 Differential expression of calbindin-D 28 kDa in rat incisor ameloblasts throughout enamel development. Anat Rec 230: 149–163.
1987 Calbindin-D28k localization in rat molar during odontogenesis. J Dent Res 66: 1431–1434.
Celio MR, Norman AW, Heizmann HW 1984 Vitamin D-dependent calcium-binding protein and parvalbumin occur in bones and teeth. Calcif Tissue Int 36: 129–130.
Taylor AN 1984 Tooth formation and the 28000 Dalton vitamin D-dependent calcium-binding protein. J Histochem Cytochem 32: 159–164.
Alvarez J 1995 Nutrition, tooth development and dental caries. Am J Clin Nutr 61: 410S–416S.
Acknowledgements
The authors appreciate the important technical assistance of D. Hotton and thank V. Hoffman and M. Ugrenovic for their technical help. We are grateful to P. Blain and C. Fondacci for providing human mandibles. We particularly thank J. Elion (director of INSERM U120 unit) for his continuing support for the dental group.
Author information
Authors and Affiliations
Additional information
Supported by Crinex Laboratories and Faculty of Dental Surgery, Paris VII University. I.B.-F. is a recipient of grants from the Fondation Goupil and an AP-HP award. P.P. is a recipient of a grant from the Fondation de France.
Rights and permissions
About this article
Cite this article
Bailleul-Forestier, I., Davideau, J., Papagerakis, P. et al. Immunolocalization of Vitamin D Receptor and Calbindin-D28k in Human Tooth Germ. Pediatr Res 39, 636–642 (1996). https://doi.org/10.1203/00006450-199604000-00013
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/00006450-199604000-00013