Abstract
Bio-based polyureas (PUs) are prepared by the polyaddition of a multifunctional bio-photodimer 4,4'-diamino-α-truxillic acid having two amines and two carboxyls with an aromatic isocyanate comonomer. Weight average molecular weights of the PUs range from 2.4 × 105 to 2.5 × 105g mol−1 with a narrow distribution around 1.3. The 10% weight-loss temperature of the PUs ranges from 270 to 280 °C, whereas the softening temperature ranges between 180 and 210 °C. The PUs are well dissolved in polar organic solvents such as dimethylformamide and the cast film is prepared from these solutions. The films show high transparency and swell in the alkaline solution when the PU carboxyls are anionized. If metal nitrate salts are added to the films, mechanical properties are increased by the double interaction of the metal cation with carboxylate and nitrate with urea, which causes the films to change the color.
Similar content being viewed by others
Introduction
Bio-based plastics are indispensable for the establishment of a green-sustainable society but the thermomechanical performance of the bioplastics currently in use is not very strong. On the other hand, aromatic polymers generally show stronger thermomechanical performance than aliphatic polymers.1, 2, 3 Recently, phenolic biomonomers were used for polyester development3, 4, 5 but the polyester films were too brittle due to low interchain interactions, and new aromatic biomonomers must be developed. We reported on the bio-based aromatic diamine 4,4'-diaminotruxillic acid (4ATA) that was photonically derived from microorganismal 4-aminocinnamic acid (4ACA). Protection of the 4ATA carboxyls by methyl groups made it possible to use 4ATA as an aromatic diamine monomer, which could be reacted with various tetraacid dianhydrides to create aromatic polyimides showing strong thermomechanical properties.6 Some of the polyimide films were transparent because of the cyclobutane ring cutting the π-conjugation between the two benzene rings. The transparent, flexible organic films with high thermal resistance are important in developing the next generation of opto-electronic materials.7, 8 However, the polyimides have a yellow tint due to slight light absorption at a wavelength of 550 nm, which is attributed to hydrogen donor–acceptor interactions on the backbone consisted of phenyl rings connecting with electron-donating nitrogen (N–H group) and electron-withdrawing carbonyl.6
Here we focus on aromatic polyureas (PUs) showing interchain hydrogen bonding, such as aromatic polyamides from 4,4'-diamino-α-truxillic acid (4ATA) whose carboxylic acids are not protected. We find that PU films are very transparent, because of a symmetrical structure connecting with the aromatic groups via the urea groups (R-NHCONH-R’), and hydrogen donor–acceptor interactions. This symmetrical structure makes the heteropolarity monomers less polar on the backbone. In addition, the PU films can be reinforced by salt addition via the double interaction of metal cation with carboxyls and counter nitrate anions with urea groups.
Experimental Procedure
Materials
4-Aminocinnamic acid (4ACA, Tokyo Chemical Industry, Tokyo, Japan), trimethylsilyl chloride (TMSCl, Sigma-Aldrich LLC, Tokyo, Japan) and anhydrous dimethyl acetamide, anhydrous (DMAc, Kanto Chemical, Tokyo, Japan) were used as received. 4,4'-diphenylmethane diisocyanate (MDI, Tokyo Chemical Industry) was purified by distillation at 160 °C under vacuum just before use. Sodium hydroxide (NaOH, Kanto Chemical), hydrochloride acid (HCl, Kanto Chemical) and methanol (Kanto Chemical) were used as received.
Monomer synthesis
4,4'-Diamino-α-truxillic acid
4,4'-Diamino-α-truxillic acid (4ATA) dihydrochloride was synthesized by the following procedure (Scheme 1). HCl aq (12 mol l−1, 3 ml) was added dropwise into a clear acetone solution (100 ml) of 4ACA (3.26 g, 20 mmol) to make the reaction solution turbid and to produce 4ACA HCl salt. HCl aq solution addition was stopped when the turbidity stopped increased. The products were collected by filtration and dried (yield; 99 wt%). 4ACA HCl salt powder (1 g) was ground to make small particles; these were dispersed in hexane (20 ml). Under magnetic agitation the dispersion was irradiated for 12 h using a high-pressure Hg lamp with the wavelength adjusted to 280–450 nm (light intensity: 285 mW cm−2) by a light filter to produce the 4ATA dihydrochloride dispersion. The completion of the photoreaction was confirmed by 1H NMR analyses of the sampled dispersants. 4ATA dihydrochloride was neutralized by the addition of NaOH aq (10 mol l−1) until the pH reached about 5, and then the precipitates of 4ATA were collected by filtration (yield: 60 wt%). The stereostructure of 4ATA was confirmed to be 4,4'-diamino-α-truxillic acid by the cyclobutane signals of 1H NMR.
4,4'-Diamino-α-truxillic acid dimethyl ester
The syntheses of 4,4'-diamino-truxillic acid methyl ester were reported.6
PU synthesis
The representative polymerization procedure of 4ATA is as follows: 4ATA (326 mg, 1 mmol) was mixed with equimolar MDI (250 mg, 1 mmol) in DMAc (5 ml) and heated to make a homogeneous solution at 50 °C in a 100-ml nitrogen-purged round-bottom flask. After the polymerization proceeded by agitation for 1 h, the reaction solution became viscous. After polymerization, the reaction solution was cooled to room temperature and was poured into ethanol to precipitate the fibrous polymers. The polymers were collected by filtration and dried in vacuo (yield; 90 wt%). The target structures of the products, poly(4,4'-truxillic acid co-methylene diphenyl) ureas (PATAMDU) were confirmed by 1H NMR and molecular weights were determined by gel permeation chromatography (GPC). The polymerization of 4ATA dimethyl ester with MDI poly(4,4'-truxillic acid methyl ester-co-methylene diphenyl) ureas (PMATAMDU) was made by the analogous procedure with 4ATA polymerization (yield: 94 wt%).
Instruments
The number average molecular weight (Mn), weight average molecular weight (Mw) and molecular weight distribution (PDI) of the polymers (concentration 5 mg l−1, filtrated after stirring over night) were determined by GPC (Shodex GPC-101 with a tandem connection column system of KD-803 and KD-807 (Shodex, Tokyo, Japan)) that was calibrated with pollutant standards at 40 °C in DMF (eluent: Dimethylformamide) at a flow rate 0.5 ml min−1 using the RI signal detector (Shodex).
1H NMR measurements were performed on a Bruker AVANCE III 400 spectrometer operating at 400.13 MHz for 1H NMR. DMSO-d6 was used as a deuterated solvent and non-deuterated DMSO included was used as an internal standard (2.5 p.p.m.). Sample concentration was 0.5 mM in DMSO-d6 as a solvent in glass test tube (Wako Pure Chemical Industries, 5.0 mmφ × 7 inch, Osaka, Japan).
FT-IR spectra were recorded with a Perkin–Elmer Spectrum One spectrometer between 4000 and 600 cm−1 using a diamond-attenuated total reflection accessory.
The ultraviolet-visible (UV-vis) transmission spectra were recorded by Perkin Elmer, Lambda 25 UV/Vis spectrophotometer at room temperature over the range of 200–800 nm in a quartz cell (1 cm × 1 cm) under Deuterium and Halogen lamp as light source.
The refractive indices of cast films were evaluated by an Abbe refractometer (Atago, NRA,1T, Tokyo, Japan) employing 1-bromonaphthalene as a contact liquid at room temperature.
Measurements of thermogravimetry and thermomechanical analysis (TMA) were performed on HITACHI STA 7200 and TMA 7100 system, respectively, under nitrogen atmosphere at a heating rate of 10 °C min−1. The polymer specimens were dried at 160 °C for 1 h to remove absorbed moisture just before measurement.
The tensile mechanical tests were carried out at an elongation speed of 0.5 mm min−1 on a tensiometer (Instron 3365, Kawasaki, Japan) at room temperature using a polymer rectangle film with a length of 2 cm, a width of 0.5 cm and a thickness of 5 μm.
Results and discussion
Synthesis
PUs with side groups of carboxylic acid or carboxylate methyl ester were prepared by the polyaddition of 4-ATA aromatic diamine or its methyl ester with stoichiometric amounts of aromatic diisocyanates MDI. The 1H NMR spectra of both PUs are shown in Figure 1, indicating that the signals for aromatics, ureas, cyclobutyl groups and methyl ester appeared around 7.0–7.5, 8.5-8.6, 3.3–4.3 and 3.2 p.p.m., respectively. The signal for the carboxylic acid of PU appeared at 12.2 p.p.m.. (FT-IR and 13C spectra in Supplementary Figure S9)
The weight average molecular weight (Mw), the number average molecular weight (Mn) and the molecular weight distribution (PDI) of the PUs were determined and are summarized in Table 1. PUs had high Mw and Mn values in ranges of 2.41–2.45 × 105 and 1.81–1.89 × 105, respectively, and the PDI value ranged between 1.28 and 1.39. The solubility of the PUs was tested using (1) non-polar solvents such as toluene, hexane, chloroform, diethyl ether and dichloromethane, (2) polar protic solvents such as distilled water, methanol and ethanol and (3) polar aprotic solvents such as acetone, acetonitrile, DMF, NMP, DMAc, DMSO, THF and ethyl acetate. The PUs were soluble in some of the polar aprotic solvents such as DMF, NMP, DMAc and DMSO at room temperature (Supplementary Table S1). The films were cast from the DMAc solution with a concentration range of 5–10 wt%, by dropping the solution on a glass and drying at 40–50 °C for 4 h or longer (Figure 2). The films were transparent, tough and flexible, and showed relatively high refractive indices of 1.61–1.63. Figures 3a and b show the UV-vis transmission spectra of PATAMDU and PMATAMDU films. The spectra were normalized at a thickness of 5 μm, and the optical transparency at 550 nm (λ550) and the cutoff wavelengths (λ0) were determined to be 91% and 310 nm for PATAMDU and 85% and 310 nm for PMATAMDU, respectively.
Thermomechanical properties
The thermal degradation of PUs was investigated by thermogravimetric analysis (representative curves in Supplementary Figure S1) in a nitrogen atmosphere at a heating rate of 10 °C min−1 and the 10% weight-loss temperature (Td10) was determined. The samples were dried at 160 °C before measurement. Td10 values were almost constant at 270–280 °C regardless of the carboxylate side group structure, which suggests that thermal degradation is regulated by backbone breakage via urea linkages. The softening temperatures (Ts) of the PUs were determined by TMA under a nitrogen atmosphere and summarized in Table 1. PATAMDU and PMATAMDU showed Ts values of 200 and 210 °C, respectively. For the thermal property, the difference of side-chain structures, carboxylic acid and methyl ester, had less effect than the same backbone structure, aromatic polyurea.
Tensile tests of the PU films were performed to understand their mechanical properties. The films of PMATAMDU and PATAMDU showed tensile strength of 21 and 17 MPa, tensile moduli of 1.2 and 1.1 GPa, and elongation at break of 2.7% and 2.1%, respectively (representative curves in Supplementary Figure S2). Density of the PMATAMDU film (1.26) was a little higher than that of the PATAMDU film (1.21).
Salt addition
PATAMDU has a hydrophilic carboxylic acid side group, which is different from previously reported polyimides or PMATAMDU.6 As water should therefore be absorbable, the water content after immersion in water was investigated. The water content of the PATAMDU film ranged from 20 to 50%, which is higher than that of the PMATAMDU film. When the pH of water was increased to 10, the water content increased to 100% of the sample weight. This phenomenon indicates that the carboxylate side group of the PU has an ionization function.
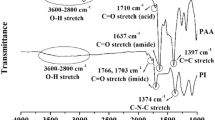
The water-swollen PATAMDU film was immersed into a standard solution of nitrate salts with heavy metals at a concentration of 1.0 mol l−1 and into diluted heavy metal solutions with concentrations of 0.1, 0.2 and 0.5 mol l−1, in order to investigate the reinforcement effect of metal ions on the films (Supplementary Table S2,Supplementary Figure S4). Figure 4 shows the molar degree of Eu salt absorption to the repeating units of the PUs. The degree of salt absorption, which was calculated by the concentration loss of the metal solution, was low (<0.2 mol/mol) in the salt concentration range of up to 1.0 mol l−1, but monotonously increased with an increase in salt concentration. In Figure 3c, transmittance spectra of the film including Eu(NO3)3 salt are shown. As a result of the salt addition, the dips of the transmittance curve were intensified around 440 and 610 nm, which might correspond to the π-π* transition of benzene rings, and λ550 decreased with an increase of salt concentration, indicating a lower transparency. In fact, the film became dark green in color by salt addition as shown in the inset picture of Figure 3c, presumably due to the nitrate hydrogen bonding with the urea group effective on the π-electron condition of benzene rings connected with the urea group.9
In order to confirm molecular adsorption based on this assumption, infrared (IR) spectra of the PATAMDU film immersed in Eu(NO3)3 aqueous solution and the PATAMDU specimen was obtained by dried samples (Supplementary Figure S8). Figure 5 (Supplementary Figure S5 and Supplementary Figure S6) shows the resulting IR spectrum together with that of pure PATAMDU film without salt and that of a mixture of PATAMDU and salt. The pure PATAMDU film and the mixture with salt showed a sharp urea IR peak at 1678 and 1605 cm−1, which belong to C=O of carboxylic acid and urea group. However, the salt-hybridized film showed different spectra; for the part of urea group that formed the hydrogen bond with nitrate anion, a new peak appeared at 1613 cm−1 from the old peak shifted, and for the carboxylic acid group absorbed the metal cation, there was a big shoulder shifted from the C=O of carboxylic acid. This reveals nitrate anion adsorption by the urea group (Figure 6).10, 11 Nitrate absorbing to urea cross-linked the PU chains and reinforced the films under the support of metal adsorption to carboxylic acid, and the increased interchain interaction caused the increase in mechanical properties.12
The optical property (λ550) and mechanical properties, such as tensile strength and tensile modulus, of the films depended on the concentration of the Eu3+ salt (Figure 7). Following the absorption of Eu3+ cation on the films, the strength of the hydrogen bond also increased, which intensified the interactions between the backbones. First, this effected a change in the distance between the backbones, which in turn caused a change in the π–electron interactions from the aromatic group between the backbones, thereby affecting the transparency of the films. Second, these effects also influenced the mechanical property of the films in that the mechanical strength and Young’s moduli increased with an increase in the amount of Eu3+ cation absorption on the films. This suggested that the metal cation adsorption had a reinforcing effect on the films. The Eu-salt addition effects on the properties of the films are summarized in Supplementary Table S2. Additional elongation at break was also increased by salt addition, presumably due to the dynamism of physical cross-linking that is capable of limiting stress concentration.
The increase in the mechanical properties is due to two effects: hydrogen bond formation between the urea group and the nitrate group and ionic interaction between the metal cation and carboxylate ions.9, 11 The versatility of the phenomenon was investigated using other metal nitrate salts with various valences such as Nd(NO3)3, Fe(NO3)2, Mg(NO3)2, In(NO3)3 and NaNO3 at the same concentration (1.0 mol l−1). The entire metal nitrate salts used here increased the mechanical properties of the films as shown in Table 2. In the constant valence, as the ionic radius decreased, the absorption amount of the metal salts decreased, directly affecting the mechanical property of the polymer film. In particular, Nd(NO3)3 had excellent effects, increasing the tensile strength to 43 MPa and tensile modulus to 1.8 GPa, respectively. Td10 was influenced very much by salt addition. On the other hand, the transmittance of the films was worse after the films absorbed the metal nitrate salts. When the PU films were immersed in these metal salts, they turned green or yellow (Supplementary Figure S3). The nitrate salt had the stronger effects on the color change comparing with chloride salt. The transmission spectrum change was confirmed (Supplementary Figure S7), which is likely caused by hydrogen bonding of nitrates to urea linkages as discussed above (Figure 6), as evidenced by IR studies. In addition, Supplementary Figure S7 also shows the optical property change at 550 nm (λ550) and the changes in cutoff wavelengths (λ0), most significant for the PU film with Nd(NO3)3.
Conclusion
We synthesized aromatic PUs with carboxylic acid. The molecular weights were high, up to 2. 5 × 105 g mol−1 and Td10 and Ts were 270 and 180 °C, respectively. The polymer films had strong mechanical properties with a tensile strength 20 MPa and tensile modulus around 1.2 GPa. The film adsorbed metal nitrate salts to strengthen the mechanical properties to 43 MPa for tensile strength and 1.8 GPa for tensile modulus. The addition of salt caused a color change to green or yellow, presumably due to nitrate hydrogen bonding with the urea linkage connecting with the benzene ring. Thus, the PUs prepared here were reinforced by the combined effects of the metal cations and nitrate anions of added salts.

Syntheses of monomers and polyureas (PUs) from photodimers with different side chains: (a) syntheses of monomers with different side chains, (b) syntheses of PUs from comonomers and MDI.
References
Mathers, R. T. & Meier, M. A. R . in Green Polymerization Methods: Renewable Starting Materials, Catalysis and Waste Reduction (eds Mathers, R. T. & Meier, M. A. R.) (Wiley-VCH Verlag GmbH & Co., Weinheim, Germany, 2011)
Zakzeski, J., Bruijnincx, P. C. A., Jongerius, A. L. & Weckhuysen, B. M. The catalytic valorization of lignin for the production of renewable chemicals. Chem. Rev. 110, 3552–3599 (2010).
Kaneko, T., Tran, H. T., Shi, D. J. & Akashi, M. Environmentally degradable, high-performance plastics from phenolic phytomonomers. Nature Mater 5, 966–970 (2006).
Miller, S. A. Sustainable polymers: opportunities for the next decade. ACS Macro Lett. 2, 550–554 (2013).
Megiatto, J. D., Cazeils, E. Jr ., Ham-Pichavant, F., Grelier, S., Gardrat, C. & Castellan, A. Styrene-spaced copolymers including anthraquinone and β-O-4 lignin model units: synthesis, characterization and reactivity under alkaline pulping conditions. Biomacromolecules 13, 1652–1662 (2012).
Suvannasara, P., Tateyama, S., Miyasato, A., Matsumura, K., Shimoda, T., Ito, T., Yamagata, Y., Fujita, T., Takaya, N. & Kaneko, T. Bio-based polyimides from 4-aminocinnamic acid photodimer. Macromelecules 47, 1586–1593 (2014).
Chopra, K. L., Major, S. & Pandya, D. K. Transparent conductors- a status review. Thin Solid Films 102, 1–46 (1983).
Ostroverkhova, O . in Handbook of Organic Materials for Optical and (Opto)- Electronic Devices (ed. Ostroverkhova, O.) (Woodhead Publishing Limited, Cambridge, UK, 2013)
Bondy, C. R., Gale, P. A. & Loeb, S. J. Metal organic analogs of Calix[4]arene based anion receptors. Arranging urea hydrogen-bond acceptors to selectively bind chloride and sulfate ions. J. Am. Chem. Soc. 126, 5030–5031 (2004).
Arman, B., Reddy, A. S. & Arya, G. Viscoelastic properties and shock response of coarse-grained models of multiblock versus diblock copolymers: insights into dissipative properties of polyurea. Macromolecules 45, 3247–3255 (2012).
Perez-Casas, C. & Yatsimirsky, A. K. Detailing hydrogen bonding and deprotonation equilibria between anions and urea/thiourea derivatives. J. Org. Chem. 73, 2275–2284 (2008).
Ferrer, A. S., Rogez, D. & Martinoty, P. Synthesis and characterization of new polyurea elastomers by sol-gel chemistry. Macromol. Chem. Phys. 211, 1712–1721 (2010).
Acknowledgements
This work was partially supported by Japan Science and Technology Agency, Advanced Low Carbon Technology Research and Development Program, No. 5170020, Tokyo, Japan.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on Polymer Journal website
Supplementary information
Rights and permissions
About this article
Cite this article
Jin, X., Tateyama, S. & Kaneko, T. Salt-induced reinforcement of anionic bio-polyureas with high transparency. Polym J 47, 727–732 (2015). https://doi.org/10.1038/pj.2015.58
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/pj.2015.58
This article is cited by
-
Syntheses of Soluble Biopolyimides Using 4-Aminophenylalanine
Chinese Journal of Polymer Science (2020)