Abstract
Stimuli-degradable cross-linked polymers were developed by applying both topological linkage and size complementarity of rotaxane to cross-link their structures. A double vinyl group-tethering [3]rotaxane cross-linker was prepared by reacting the axle ends of sec-ammonium/crown ether-type pseudo[3]rotaxane with a suitably bulky end-cap agent that was as large as the cavity of the wheel. Radical polymerization of a common vinyl monomer in the presence of the [3]rotaxane cross-linker afforded the corresponding stable cross-linked polymer under ambient conditions. An anion exchange reaction with tetra(n-butyl)ammonium chloride caused selective and efficient de-cross-linking of the cross-linked polymers to the vinyl polymer and the axle component of the cross-linker.
Similar content being viewed by others
Introduction
Chemical recycling of cross-linked polymers is an important current issue that needs to be solved in the area of polymer science and technology.1, 2, 3 The use of stimuli-degradable cross-linked polymers, which is one of the most promising approaches, often requires a special dynamic property at the cross-link point that has been primarily achieved by three approaches. The first approach involves the utilization of the cross-linking reaction based on dynamic covalent chemistry4, 5, 6, 7, 8 using reversible reactions, such as imine formation9, 10, 11 and the Diels–Alder reaction.12 The second approach involves formation of the cross-link point using intermolecular interactions, such as hydrogen bonding.13, 14 Although polymers with supramolecular cross-links (that is, physical cross-links) readily undergo de-cross-linking under mild conditions, this approach is limited due to weak stability. The third approach involves the utilization of both mechanical linkage, such as rotaxane linkage, and dynamic covalent chemistry or size complementarity of the linkage. We have used a thiol-disulfide interchange reaction at the rotaxane cross-link points for the construction and degradation of cross-linked polymers, thus revealing a novel concept for the chemical recycling of the cross-linked polymer.15, 16 In addition, the size-complementary rotaxane, which has an axle whose size is similar to that of the wheel cavity, was employed as the cross-link point for the stimuli-degradable cross-linked polymer.17 However, in these systems, we had to use main chain-type poly(crown ether)s as the trunk polymers. Therefore, trunk polymer versatility was not achieved. To achieve polymer versatility, we developed a vinylic rotaxane cross-linker that yields stimuli-degradable polymers via the radical polymerization of a variety of vinyl monomers to prove the usefulness of this protocol. In this study, we describe our novel concept for producing stimuli-degradable cross-linked polymers obtained by the radical polymerization of common vinyl monomers in the presence of a size-complementary [3]rotaxane cross-linker with two vinyl groups.18
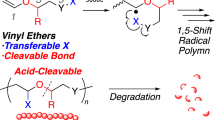
The strategy of the present approach for the synthesis and degradation of cross-linked polymers is illustrated in Figure 1, where the key material is a size-complementary vinylic [3]rotaxane cross-linker possessing the bulky axle end-cap group that is as large as the cavity of the wheel component. Simple vinyl polymerization of the monomer in the presence of the cross-linker affords the corresponding rotaxane cross-linked polymer (RCP) (For a related report, see Arai et al.19). The energy barrier to deslip the axle component of the rotaxane moiety of the RCP is high enough to obtain a stable cross-linked polymer under ordinary conditions.20, 21, 22, 23, 24 Meanwhile, the [3]rotaxane moiety that links the polymer chains in the RCP undergoes degradation via deslippage by a stimulus capable of breaking off the attractive interaction between the wheel and axle components. Therefore, we can obtain soluble polymer by the simple degradation of the RCP obtained with the stimuli-degradable rotaxane cross-linker. As radical polymerization can be used in this approach, a variety of vinyl monomers can be employed.
Representation of the stimuli-degradable cross-linked polymer prepared by radical polymerization of a vinyl monomer in the presence of a [3]rotaxane cross-linker and its degradation via deslippage of the size-complementary [3]rotaxane moiety due to chemical stimuli. A full color version of this figure is available at Polymer Journal online.
Materials and methods
Materials
CH2Cl2 was dried over freshly activated molecular sieves 4A (MS 4A). The other commercially available reagents and solvents were used without further purification, unless otherwise noted. Wako Gel (Wako Pure Chemical Industries, Ltd., Osaka, Japan) was used for silica gel column chromatography. Axles 1 and 2 were synthesized according to previously published protocols.17, 21
Synthesis of hydroxymethyl dibenzo-24-crown-8-ether (DB24C8) containing methacrylate 3
To a solution of hydroxymethyl dibenzo-24-crown-8-ether (1.92 g, 3.03 mmol) in dichloromethane (45 ml), 2-isocyanatoethyl methacrylate (1.35 ml, 9.54 mmol) and dibutyltin dilaurate (183 μl, 0.297 mmol) at 0 °C were added, and the mixture was stirred for 48 h at ambient temperature. The mixture was concentrated to 10 ml, followed by precipitation in 100 ml of hexane. The residual mixture was filtered and then subjected to purification using silica gel column chromatography (hexane:AcOEt=2:3→AcOEt) to produce the titled compound as a white solid (2.45 g, 2.91 mmol) with a 96% yield.
Synthesis of size-complementary [3]rotaxane 4
Axle 1 (0.21 g, 0.60 mmol) was mixed with wheel 3 (0.38 g, 0.60 mmol) in dichloromethane (3 ml), and the mixture was stirred for 3 h to form a pseudorotaxane. Then, methylene diphenyl diisocyanate (75 mg, 0.30 mmol) and dibutyltin dilaurate (23 μl, 37 μmol) were added to the mixture at 0 °C. After stirring for 12 h at ambient temperature, the solvent was removed under reduced pressure. The residual mixture was subjected to purification using silica gel column chromatography (CHCl3:MeOH=30:1) to produce the titled compound as a white solid (0.47 g, 0.21 mmol) with a 71% yield (Scheme 1).
Synthesis of [3]rotaxane 5
Axle 2 (60 mg, 0.15 mmol) was mixed with wheel 3 (95 mg, 0.15 mmol) in dichloromethane (0.75 ml), and the mixture was stirred for 3 h to form a pseudorotaxane. Then, methylene diphenyl diisocyanate (19 mg, 75 μmol) and dibutyltin dilaurate (5.7 μl, 9.2 μmol) were added to the mixture at 0 °C. After stirring for 12 h at ambient temperature, the solvent was removed under reduced pressure. The residual mixture was subjected to purification using high-performance liquid chromatography to produce the titled compound as a white solid (100 mg, 43 μmol) with a 57% yield.
Typical procedure for the preparation of RCPs
Methyl methacrylate (0.51 ml, 4.8 mmol), azobisisobutyronitrile (7.9 mg, 48 μmol) and [3]rotaxane 4 (108 mg, 48 μmol) were dissolved in N,N-dimethylformamide (0.48 ml) and then degassed three times via the freeze thaw technique. The mixture was placed into a polypropylene-sealed tube, and then the tube was maintained at 60 °C for 12 h. The obtained gel was purified by repeated swelling in chloroform and methanol, followed by gentle drying at room temperature for 1 day and in vacuo for 1 day to produce the corresponding polyrotaxane network RCP-1 (0.576 g, 97%).
Typical procedure for de-cross-linking of RCPs
RCP-1 (10.6 mg) was added to a solution of tetra(n-butyl)ammonium chloride (Bu4NCl) (195 mg, 0.70 mmol) in N,N-dimethylformamide (7 ml) and warmed at 60 °C. After solubilization of the gel for 12 h, the mixture was concentrated and poured into 25 ml of methanol. The obtained white solid was filtered and dried in vacuo to quantitatively yield copolymer 7. The 1H NMR (nuclear magnetic resonance) spectrum of copolymer 7 indicates that the polymer is composed of Poly(methyl methacrylate) and 3.
Re-cross-linking from copolymer 7
To a solution of copolymer 7 (300 mg) in CH2Cl2 (500 μl), axle 1 (9.1 mg, 17.7 μmol) was added, and the mixture was stirred for 3 h to form a pseudorotaxane. Then, methylene diphenyl diisocyanate (3.3 mg, 8.9 μmol) and dibutyltin dilaurate (5 μl, 8 μmol) were added to the mixture at 0 °C and stirred for 12 h to obtain the re-cross-linked polymer (RCP-R) as a white solid.
Characterization
1H (400 MHz) and 13C (100 MHz) NMR spectra were recorded on a JEOL AL-400 spectrometer using CDCl3 and dimethyl sulfoxide-d6 as the solvents and calibrated using the residual undeuterated solvent and tetramethylsilane as the internal standard. The infrared (IR) spectra were recorded on a JASCO FT/IR-230 spectrometer. The melting points were measured on a MELTING POINT APPARATUS SMP3 (Stuart Scientific, Staffordshire, UK) instrument. Fast-atom bombardment and electrospray-ionization high-resolution mass spectrometry) spectra were obtained at the Center for Advanced Material Analysis, Tokyo Institute of Technology. Thermogravimetric analysis (TGA) was performed on a Shimadzu TGA-50 instrument under an N2 atmosphere (flow rate of 50 ml min−1) to determine the 5% weight decomposition temperature (Td5) at which 5% weight loss was observed. Differential scanning calorimetry (DSC) analyses were performed with a Shimadzu DSC-60 instrument with an N2 atmosphere (flow rate of 50 ml min−1) to determine the glass transition temperature (Tg). Preparative gel permeation chromatography was performed using a HPLC LC-918 instrument obtained from Japan Analytical Industry (Tokyo, Japan) with a Megapak-Gel 201C. Dynamic mechanical analysis was performed on an IT-DVA200s (ITK, Osaka, Japan) apparatus.
Results and Discussion
Deslippage of size-complementary [3]rotaxane
First, we investigated the synthesis of the size-complementary [3]rotaxane cross-linker (4) and the model degradation of 4 via deslippage. [3]Rotaxane 4, with tBu groups as the suitable size-complementary axle end groups to the DB24C8 wheel, was designed and synthesized according to a typical threading-end-capping protocol,22, 23 in which the initial threading of tBu and hydroxy-terminated sec-ammonium axle (1) with methacryloyloxy DB24C8 (3) with pseudorotaxane was followed by dimerization using a diisocyanate. [3]Rotaxane 5, which contained two 3,5-dimethylphenyl end groups that are bulky enough to prevent deslippage, was also prepared in a similar manner as a reference compound. The structures of these rotaxanes were determined using 1H NMR, 13C NMR, IR and electrospray-ionization high-resolution mass spectrometry spectra.
To clarify the effect of size complementarity on degradation, 4 and 5 were subjected to a dissociation experiment. As this type of size-complementary rotaxane is stabilized not only by size-complementary components but also by strong hydrogen bonding interaction between the components, understanding how to decrease the hydrogen bonding interaction is the key for effective deslippage. Next, we decided to use the counter anion exchange reaction of the ammonium salt moiety, which is based on the hard soft acid base principle25 and markedly decreases the acidity of the ammonium moiety, according to our neutralization method developed for the crown/ammonium-type rotaxane.26
First, we selected tetra(n-butyl)ammonium fluoride (Bu4NF) for the dissociation of 4. Treatment with 50 eq. of Bu4NF in dimethyl sulfoxide-d6 at 60 °C resulted in the decomposition of 4. Based on the 1H NMR spectrum of the reaction mixture, Bu4NF caused unexpected ester hydrolysis of the wheel component in addition to the formal deslippage of 4 to the axle component of 4 and wheel 3. This result is most likely due to the relatively strong basicity of Bu4NF. To avoid such a side reaction, we chose to use a chloride anion instead of a fluoride anion (that is, Bu4NCl as an external stimulus). The reaction of 4 with Bu4NCl (50 eq.) in dimethyl sulfoxide-d6 at 60 °C smoothly proceeded to yield the two corresponding components (that is, wheel 3 and axle component 6) without any decomposition of the framework (Scheme 2). However, similar treatment of 5 with Bu4NCl resulted in no product, which strongly indicated the marked effect of size complementarity in addition to the dissociation process of 4 via deslippage.
Synthesis of stimuli-degradable cross-linked polymers
Based on the above results, we examined the synthesis and the degradability of RCP. [3]Rotaxane cross-linkers 4 and 5 were employed as cross-linkers by addition to the free radical polymerization system of methyl methacrylate or n-butyl acrylate, which are typical vinyl monomers (Scheme 3). A mixture of a vinyl monomer, 4 or 5 (1–2 mol%), and azobisisobutyronitrile (1 mol%) was heated to 60 °C for 12 h to afford a gelled product, which strongly indicated that 4 and 5 work as cross-linkers of the vinyl polymer. The products were purified by swelling in CHCl3 and MeOH to remove the unreacted materials and gently dried at room temperature for 1 day and in vacuo for 1 day to yield RCP-1–4 in high yields (Table 1, Figure 4a).
To evaluate the chemical stability of the RCPs, they were soaked in chloroform, toluene, methanol, water, and DMF for 1 d to yield sufficiently swollen organo gels. It was noted that the swollen gel maintained its shape even in N,N-dimethylformamide (DMF) after an additional day without any degradation, as shown in Figure 4(b), which demonstrated the sufficiently high stability of the RCPs derived from the rotaxane cross-linker 4 with stimuli-degradability.
Figure 2 shows the dynamic mechanical analysis of the dried RCP-3 film. The tensile storage modulus (E′) below the glass transition temperature at –44.1 °C was recorded as >109Pa. In addition, the plateau regions of the tensile storage modulus ranging from 0 to 150 °C clearly indicated a cross-linked structure for RCP-3. The dynamic mechanical analysis profile of RCP-3 was in good agreement with that of covalently cross-linked poly(n-butyl acrylate), which has a similar degree of cross-linking and was prepared using a bifunctional diacrylate (See Supplementary Materials), implying that RCP-3 should exhibit physical stability as high as that of the cross-linked poly(n-butyl acrylate).
Next, we investigated the stimuli degradability of RCPs according to the results of the above model reaction of 4. The addition of Bu4NCl to the gels of RCPs 1, 2 and 3 swollen in DMF at 60 °C caused effective and selective degradation to afford a clean solution that contained linear polymer and the axle components in quantitative yields (Scheme 4, Figure 4c). In addition, RCP-4 was very stable and maintained its shape in Bu4NCl. The results clearly indicated excellent stimuli degradability based on the size complementarity of 4.
The size exclusion chromatography result of the MeOH-insoluble polymer from RCP-1 resulted in an Mn of 120 kg mol−1 and Mw/Mn of 2.0. The 1H NMR spectrum of copolymer 7 (Figure 3) confirmed the formation of poly(methyl methacrylate) with a small amount of the DB24C8 moiety with a composition corresponding to the feed ratio (ca. 99:1).
Finally, recovered copolymer 7 was subjected to a re-cross-linking reaction using axle 1 and methylene diphenyl diisocyanate in dichloromethane for 24 h. Additional dichloromethane was poured into the flask, and a swollen product (RCP-R) was obtained, as shown in Figure 4d.
Conclusions
In conclusion, we have demonstrated a new concept for the construction of a stimuli-degradable cross-linked polymer using the characteristics of both the rotaxane cross-link and size-complementary rotaxane. The high recovery yield of the trunk polymer demonstrates the potential usefulness of this concept as a reasonable method for recycling cross-linked polymers.

A full color version of this figure is available at Polymer Journal online.

A full color version of this figure is available at Polymer Journal online.

A full color version of this figure is available at Polymer Journal online.

A full color version of this figure is available at Polymer Journal online.
References
Endo, T. & Sanda, F. Molecular design of novel network polymers. Angew. Makromol. Chem. 240, 171–180 (1996).
Sanda, F. & Endo, T. Cationic equilibrium ring-opening polymerisation of bicyclic monomers and its application to chemical recycling of the new polymer material. Polym. Recycl. 3, 159–163 (1998).
Sanda, F. & Endo, T. Development of polymeric materials applicable to chemical recycling. Eco. Ind. 6, 18–26 (2001).
Kloxin, C. J., Scott, T. F., Adzima, B. J. & Bowman, C. N. Covalent adaptable networks (CANs): a unique paradigm in cross-linked polymers. Macromolecules 43, 2643–2653 (2010).
Maeda, T., Otsuka, H. & Takahara, A. Dynamic covalent polymers: reorganizable polymers with dynamic covalent bonds. Prog. Polym. Sci. 34, 581–604 (2009).
Endo, T. & Sudo, A. Development and application of novel ring-opening polymerizations to functional networked polymers. J. Polym. Sci. Part A: Polym. Chem. 47, 4847–4858 (2009).
Bergman, S. D. & Wudl, F. Mendable polymers. J. Mater. Chem. 18, 41–62 (2008).
Lehn, J. M. Dynamers: dynamic molecular and supramolecular polymers. Prog. Polym. Sci. 30, 814–831 (2005).
Mohr, G. J., Tirelli, N., Lohse, C. & Spichiger-Keller, U. Development of chromogenic copolymers for optical detection of amines. Adv. Mater. 10, 1353–1357 (1998).
Oh, K., Jeong, K. S. & Moore, J. S. m-Phenylene ethynylene sequences joined by imine linkages: dynamic covalent oligomers. J. Org. Chem. 68, 8397–8403 (2003).
Ono, T., Fujii, S., Nobori, T. & Lehn, J. M. Soft-to-hard transformation of the mechanical properties of dynamic covalent polymers through component incorporation. Chem. Commun. 46–48 (2007).
Chen, X., Dam, M. A., Ono, K., Mal, A., Shen, H., Nutt, S. R., Sheran, K. & Wudl, F. A thermally re-mendable cross-linked polymeric material. Science 295, 1698–1702 (2002).
Binder, W. H. & Zirbs, R. Supramolecular polymers and networks with hydrogen bonds in the main- and side-chain. Adv. Polym. Sci. 207, 1–78 (2007).
ten Brinke, G., Ruokolainen, J. & Ikkala, O. Supramolecular materials based on hydrogen-bonded polymers. Adv. Polym. Sci. 207, 113–177 (2007).
Oku, T., Furusho, Y. & Takata, T. A concept for recyclable cross-linked polymers: Topologically networked polyrotaxane capable of undergoing reversible assembly and disassembly. Angew. Chem Int. Ed. Engl. 43, 966–969 (2004).
Bilig, T., Oku, T., Fursho, Y., Koyama, Y., Asai, S. & Takata, T. Polyrotaxane networks formed via rotaxanation utilizing dynamic covalent chemistry of disulfide. Macromolecules 41, 8496–8503 (2008).
Kohsaka, Y., Nakazono, K., Koyama, Y., Asai, S. & Takata, T. Size-complementary rotaxane cross-linking for the stabilization and degradation of a supramolecular network. Angew. Chem. Int. Ed. Engl. 50, 4872–4875 (2011).
Koyama, Y., Yoshii, T., Kohsaka, Y. & Takata, T. Photodegradable cross-linked polymer derived from a vinylic rotaxane cross-linker possessing aromatic disulfide axle. Pure Appl. Chem. 85, 835–842 (2013).
Arai, T., Jang, K., Koyama, Y., Asai, S. & Takata, T. Versatile supramolecular cross-linker: rotaxane cross-linker that directly endows vinyl polymers with movable cross-links. Chem. Eur. J. 19, 5917–5923 (2013).
Ashton, P. R., Baxter, I., Fyfe, M. C. T., Raymo, F. M., Spencer, N., Stoddart, J. F., White, A. J. P. & Williams, D. J. Rotaxane or pseudorotaxane? That is the question!. J. Am. Chem. Soc. 120, 2297–2307 (1998).
Tachibana, Y., Kawasaki, H., Kihara, N. & Takata, T. Sequential O- and N-acylation protocol for high-yield preparation and modification of rotaxanes: synthesis, functionalization, structure, and intercomponent interaction of rotaxanes. J. Org. Chem. 71, 5093–5104 (2006).
Makita, Y., Kihara, N. & Takata, T. Quantitative active transport in [2]Rotaxane using a one-shot acylation reaction toward the linear molecular motor. J. Org. Chem. 73, 9245–9250 (2008).
Tachibana, Y., Kihara, N., Furusho, Y. & Takata, T. Is the tert-Butyl group bulky enough to end-cap a pseudorotaxane with a 24-crown-8-ether wheel? Org. Lett. 6, 4507–4509 (2004).
Akae, Y., Okamura, H., Koyama, Y., Arai, T. & Takata, T. Selective synthesis of [3]Rotaxane consisting of size-complementary components and its stepwise deslippage. Org. Lett. 14, 2226–2229 (2012).
Pearson, R. G. Hard and soft acids and bases. J. Am. Chem. Soc. 85, 3533–3539 (1963).
Nakazono, K. & Takata, T. Neutralization of a sec-ammonium group unusually stabilized by the “rotaxane effect”: synthesis, structure, and dynamic nature of a “free” sec-amine/crown ether-type rotaxane. Chem.-Eur. J. 16, 13783–13794 (2010).
Acknowledgements
This work was financially supported by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology, Japan (No. 23245031).
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on Polymer Journal website
Supplementary information
Rights and permissions
About this article
Cite this article
Iijima, K., Kohsaka, Y., Koyama, Y. et al. Stimuli-degradable cross-linked polymers synthesized by radical polymerization using a size-complementary [3]rotaxane cross-linker. Polym J 46, 67–72 (2014). https://doi.org/10.1038/pj.2013.63
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/pj.2013.63