Abstract
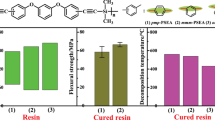
A series of silicon-containing aromatic bispropargyl ether resins—poly(dimethylsilylene-co-propynylene ether resorcinol) [–Si(CH3)2–C≡C–CH2–O–Ar–O–CH2–C≡C–]n (polymer a), poly(dimethylsilylene-co-propynylene ether bisphenol A) [–Si(CH3)2–C≡C–CH2–O–Ar–C(CH3)2–Ar–O–CH2–C≡C–]n (polymer b) and poly(dimethylsilylene-co-propynylene ether hexafluorobisphenol A) [–Si(CH3)2–C≡C–CH2–O–Ar–C(CF3)2–Ar–O–CH2–C≡C–]n (polymer c)—were synthesized from bispropargyl ethers bisphenol and dimethyldichlorosilane by a Grignard reaction. The polymers were characterized by Fourier transform infrared, hydrogen-1 nuclear magnetic resonance, gel-permeation chromatography, rheological analysis, differential scanning calorimetry and thermogravimetric analysis. The results showed that the obtained polymers could be cured at ∼240 °C and displayed good heat resistance and processing properties. Degradation temperatures of the three cured polymers at 5% weight loss (Td5) were above 400 °C, and thermal stabilities increased in the order polymer c<polymer b<polymer a. Carbon fiber (T300)-reinforced composites were prepared by a solution-impregnation process using tetrahydrofuran as solvent. The flexural strength, flexural modulus and shear strength of the composites of polymer c reached 408 MPa, 53.3 GPa and 24.8 MPa, respectively, which were higher than those of polymers a and b.
Similar content being viewed by others
Introduction
In recent years, many studies have been devoted to the synthesis of silicon-containing polymers because of their potential applications in areas such as coatings, composite matrices, ceramic precursors, optical materials and semi-conducting materials.1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11 Some silicon-containing thermosetting polymers with ethynyl or ethynylene groups have been explored and developed. Itoh et al.12, 13, 14, 15 prepared poly(phenylsilyleneethynylene-1,3-phenyleneethynylene) [–Si(Ph)H–C≡C–C6H4–C≡C–]n (abbreviated MSP) by a dehydrogenative coupling polymerization reaction between phenylsilane and m-diethynylbenzene in the presence of magnesium oxide. The decomposition temperature of the cured polymer at 5% weight loss (Td5) was above 860 °C, and the residue yield at 1000 °C was 94%. Buvat et al.16, 17 synthesized poly(silyleneethynylene-phenyleneethynylene) terminated with phenylacetylene (abbreviated as BLJ) on the basis of MSP. The BLJ resin had good processability and high heat resistance. In our laboratory, Wang et al.18 prepared poly(dimethylsilyleneethynylene-phenyleneethynylene) terminated with phenylacetylene, and the results showed that the resin had good processing performance and high thermal stability.
However, the high cross-linking density of cured silicon-containing polymers with [–SiR2–C≡C–] and [–SiR2–C≡C–Ar–C≡C–] units (R=alkyl or phenyl) resulted in low toughness, and the embrittlement influenced the mechanical properties of the composites. In addition, these polymers were prepared from expensive diyne monomers. High price and low toughness have limited the development and applications of these polymers and their relevant products. The basic polymer structure design was an important factor in improving the toughness. In this paper, three kinds of low-cost aromatic bispropargyl ethers (namely bispropargyl ether resorcin, bispropargyl ether bisphenol A and bispropargyl ether hexafluorobisphenol A) were used as diyne monomers to prepare novel silicon-containing aromatic bispropargyl ether polymers, poly(dimethylsilylene-co-propynylene ether bisphenol) [–Si(CH3)2–C≡C–CH2–O–R–O–CH2–C≡C–]n (R=Ar, Ar–C(CH3)2–Ar and Ar–C(CF3)2–Ar). The flexible units –Ar–O–CH2– in the main chains of the polymers should enhance the mechanical properties of the composites compared with those of silicon-containing arylacetylene polymers.
Experimental procedure
Materials
Tetrahydrofuran (THF) (chemical purity) and toluene (analytical reagent) were dried over sodium and distilled before use. Ethyl bromide (analytical reagent) and dimethyldichlorosilane were distilled before use. Magnesium powder was used as purchased, without any treatment. All of the materials mentioned above were purchased from Sinopharm Chemical Reagent (Shanghai, China). Carbon fibers (two-dimensional fabric, plain, T300) were purchased from Toray Company (Tokyo, Japan). Bispropargyl ether bisphenol A, bispropargyl ether hexafluorobisphenol A and bispropargyl ether resorcin were synthesized according to the literature.19
Instruments
The Fourier transform infrared (FT-IR) spectra of the polymers were collected using a Nicolet 5700 infrared spectrophotometer (Thermo Electron Scientific Instruments Corporation, Waltham, MA, USA). The hydrogen-1 nuclear magnetic resonance (1H-NMR) spectrum was measured in CDCl3 solution at 500 MHz using an AVANCE 500 spectrometer (Bruker BioSpin GmbH, Rheinstetten, Germany) using tetramethylsilane as an internal standard. Rheological behavior was traced on a RheoStress RS600 rheometer (Thermo Haake Corporation, Paramus, NJ, USA) at a heating rate of 2 °C min−1 and a shear rate of 0.1 s−1. Differential scanning calorimetry (DSC) was conducted using a 200-PC temperature-modulated system (NETZSCH-Gerätebau GmbH, Selb, Germany) with a heating rate of 10 °C min−1 and a nitrogen flow rate of 15 cm3 min−1. Thermogravimetric analyses were conducted on a thermogravimetric analysis/SDTA 851 analyzer (Mettler-Toledo GmbH, Greifensee, Switzerland) under nitrogen at a heating rate of 10 °C min−1. The flexural and shear tests of the composites were conducted using DXLL-5000 Universal Tension Tester (Shanghai De Jie Instrument Equipment, Shanghai, China) according to the China State Standard GB/T3356-1999.
Synthesis of poly(dimethylsilylene-co-propynylene ether bisphenol)
The entire reaction process was carried out in dry nitrogen. Magnesium powder (14.40 g, 0.60 mol) and THF (150 ml) were added to a 1000-ml four-neck round-bottom flask equipped with a condenser, a thermometer, a constant-pressure dropping funnel and a stirrer. To the flask, ethyl bromide (56.68 g, 0.52 mol) in THF (50 ml) was added drop-wise for 1 h, and then the mixture was refluxed for 1.5 h to produce ethylmagnesium bromide. Thereafter, the reaction system was cooled to room temperature, and a solution of bispropargyl ether bisphenol (0.25 mol) in THF (150 ml) was added over 2 h with stirring. The reaction was continued for an additional 3 h while refluxing to produce the expected organic magnesium Grignard reagents.
Next, a solution of dimethyldichlorosilane (21.93 g, 0.17 mol) in THF (50 ml) was drop-wise added to the flask at room temperature over 1 h, and the reaction system was further refluxed for another 4 h. Thereafter, a solution of acetic acid (30.00 g, 0.50 mol) in toluene (150 ml) and 150 ml 2% aqueous hydrochloric acid were drop-wise added to the flask, which was placed in an ice water bath to cool. The resulting oil phase was separated out, washed with de-ionized water and then dried using anhydrous sodium sulfate. The solvent was removed in vacuo to obtain a viscous brown liquid with 87–93% yield. The synthesis route to silicon-containing aromatic bispropargyl ether resins is shown in Figure 1.
1H-NMR (δ/p.p.m.): Polymer a: 0.39 (s, 6H, Si–CH3), 2.53 (s, 1H, C≡CH), 4.68 (s, 2H, –CH2–), 6.60 (s, 3H, Ar–H), 7.20 (s, 1H, Ar–H). Polymer b: 0.32 (s, 6H, Si–CH3), 1.63 (s, 6H, C–CH3), 2.51 (s, 1H, C≡CH), 4.65 (s, 2H, –CH2–), 6.88 (s, 2H, Ar–H), 7.13 (s, 2H, Ar–H). Polymer c: 0.33 (s, 6H, Si–CH3), 2.54 (s, 1H, C≡CH), 4.71 (s, 2H, –CH2–), 6.97 (s, 2H, Ar–H), 7.32 (s, 2H, Ar–H).
FT-IR (cm−1): Polymer a: 3289 (≡C–H), 2963(–CH3), 2178 (–C≡C–), 1596, 1488 and 1453 (–Ar–), 1257 (Si–CH3), 1149 (–Ar–O–), 1043 (–O–CH2–). Polymer b: 3287 (≡C–H), 2967 (–CH3), 2183 (–C≡C–), 1607, 1508 and 1451 (–Ar–), 1252 (Si–CH3), 1223 (–Ar–O–), 1183 (CH3–C–CH3), 1037 (–O–CH2–). Polymer c: 3297 (≡C–H), 2966 (–CH3), 2185 (–C≡C–), 1611, 1513 and 1452 (–Ar–), 1252 (Si–CH3), 1205 (–Ar–O–), 1174 (CF3–C–CF3), 1038 (–O–CH2–).
Preparation of cured poly(dimethylsilylene-co-propynylene ether bisphenol)
Three silicon-containing aromatic bispropargyl ether resins were cured in air inside a temperature-controlled oven first for 2 h at 210 °C, then for 2 h at 240 °C, for 2 h at 270 °C and finally for 4 h at 310 °C. Polymer b was post-cured for an additional 4 h at 340 °C, and polymer c for an additional 2 h at 330°C and for 4 h at 360 °C. The cured resins were dense black solids.
Preparation of composites
Silicon-containing aromatic bispropargyl ether polymer/carbon fabric prepreg was prepared by a solution-impregnation process using THF as the solvent. Carbon fibers (two-dimensional fabric, plain, T300) were impregnated with silicon-containing aromatic bispropargyl ether resin solution (37 wt% resin in THF) and dried in air for 6–10 h. Thereafter, the prepreg sheets were stacked and further dried in a vacuum oven to remove the solvent. The piled prepregs of polymers a, b and c were placed in a preheated mold and pressed under a constant pressure of 3 MPa for 2 h at 210 °C, for 2 h at 240 °C, for 2 h at 270 °C and for 4 h at 310 °C. The laminate of polymer b was further post-treated under the same pressure for 4 h at 340 °C, and likewise polymer c for 2 h at 330 °C and 4 h at 360 °C. Finally, the composites were allowed to cool naturally to room temperature, and high-quality composite laminates without voids were obtained by prepreg consolidation.
Results and Discussion
Structure characterization of poly(dimethylsilylene-co-propynylene ether bisphenol)
Poly(dimethylsilylene-co-propynylene ether resorcinol) (polymer a), poly(dimethylsilylene-co-propynylene ether bisphenol A) (polymer b) and poly(dimethylsilylene-co-propynylene ether hexafluorobisphenol A) (polymer c) were analyzed by GPC, 1H-NMR and FT-IR, respectively. The GPC analysis results are presented in Table 1. As shown in the table, the molecular weights (Mn) of polymers a, b and c range from 1378 to 1668 with polydispersity index between 1.39 and 2.15.
The FT-IR spectra of poly(dimethylsilylene-co-propynylene ether bisphenol) are given in Figure 2. The three silicon-containing aromatic bispropargyl ether resins contained some identical groups with similar absorption locations. All resins featured absorptions near 3290 cm−1 (≡C–H), 3080 cm−1 (Ar–H), 2965 cm1 (–CH3), 2180 cm1 (–C≡C–), 1255 cm−1 (Si–CH3), 1205 cm−1 (–Ar–O–) and 1040 cm−1 (–O–CH2–). In addition, adsorptions at 1183 cm−1 in the FT-IR spectra of polymer b and at 1174 cm−1 in the spectra of polymer c were attributed to CH3–C–CH3 and CF3–C–CF3 skeleton-stretching vibrations, respectively.
The 1H-NMR spectra of poly(dimethylsilylene-co-propynylene ether bisphenol) are shown in Figure 3. The three silicon-containing aromatic bispropargyl ether resins contained some identical groups with similar resonance peaks in the 1H-NMR spectra. The resonance peaks at 6.60–7.32 p.p.m. were assigned to the aromatic protons (Ar–H), whereas the peaks near 4.70 p.p.m. were caused by the methylene groups (–CH2–). The peaks of ethynyl groups (–C≡C–H) appeared near 2.52 p.p.m. and those of the silane units (–Si(CH3)2–) between 0.32 and 0.39 p.p.m. The peak at 1.63 p.p.m. in the spectrum of polymer b was attributed to –C(CH3)2– in the backbone.
Rheological behavior of poly(dimethylsilylene-co-propynylene ether bisphenol)
The viscosity of each resin was measured using a rheometer. As seen in Figure 4, the viscosity showed an obvious initial decrease as the temperature increased, then stabilized and finally increased dramatically when the temperature reached a certain value. Polymers a, b and c had different melting temperatures. Polymer a melted at 61 °C, polymer b at 85 °C and polymer c at 93 °C. Melted polymer a had a viscosity of 5.23 Pa s, whereas for polymer b it was 4.90 Pa s and for polymer c 6.26 Pa s. Gelation temperatures were also different. Curing reactions of polymers a, b and c took place at 240, 245 and 249 °C, respectively, when gelation occurred. Accordingly, the processing windows of polymers a, b and c were 61–240 °C, 85–245 °C and 93–249 °C, respectively. In conclusion, the curing reaction activities of silicon-containing aromatic bispropargyl ether resins were affected by the different structures of aromatic bispropargyl ethers in their backbones.
The curing reaction of poly(dimethylsilylene-co-propynylene ether bisphenol)
The curing behaviors of the three polymers were investigated by DSC under nitrogen at a heating rate of 10 °C min−1. The corresponding DSC thermograms are displayed in Figure 5. The temperature of cure initiation for polymer a was 240 °C, whereas for polymer b it was 248 °C and for polymer c 258 °C. It was observed that polymer a showed a unimodal reaction exotherm, whereas polymers b and c showed double-peaked exotherms. It was assumed that two reactions occurred during the curing process. The exotherm at the lower temperature in the DSC curves was attributed to the rearrangement of terminal propargyl ether groups into a chromene ring,20, 21, 22, 23, 24, 25 whereas the exotherm at the higher temperature was caused by the cross-linking reactions of internal alkyne groups in the polymer.26 The DSC exotherm for polymer a peaked at 276 °C. The DSC exotherms at the lower temperatures for polymers b and c peaked at 288 and 297 °C, respectively. The rearrangement of the propargyl ether groups into a chromene ring was electrophilic in nature, and it was retarded by the presence of electron-withdrawing groups on the benzene ring. The perfluoroisopropylidene group (in polymer c) was more electron withdrawing than was the isopropylidene group (in polymer b), which in turn, was more electron withdrawing than was the propargyl ether group (in polymer a); hence, the onset and peak temperatures in the rearrangement reactions of polymers a, b and c increased in turn. As shown in Figure 5, the DSC exotherms for polymers a, b and c peaked at 276, 313 (higher temperature) and 332 °C (higher temperature), respectively, and were mainly affected by the cross-linking reactions of the internal alkyne groups. The steric hindrance of resorcinol (in polymer a), isopropylidene group (in polymer b) and perfluoroisopropylidene group (in polymer c) increased in turn, which greatly influenced the cross-linking reactions of the internal alkyne groups. Therefore, the peak temperature of the cross-linking reaction of the internal alkyne of polymer c was higher than that of polymer b, which in turn was higher than that of polymer a. In particular, the steric hindrance of resorcinol was much smaller than that of the other two, and the exotherm peak representing the rearrangement of the terminal propargyl ether groups into a chromene ring overlapped that representing the internal alkyne reaction. Thus, polymer a showed a unimodal reaction exotherm. The exothermic heats (ΔH) of curing of polymers a, b and c were 1251, 420 and 618 J mol−1, respectively, depending on the structures of the aromatic bispropargyl ethers.
Polymers a, b and c were heat treated under different isothermal conditions depending on the different structures of the polymers. The heat-treated samples were analyzed with liquid-state 1H-NMR spectroscopy. For all the polymers studied in this paper, the same types of reaction products formed in the curing mixture. Thus, from a mechanistic point of view, we present the result obtained from polymer c as an example. Polymer c was heat treated at 210 °C for 3 h. The possible reaction occurring in the first stage of the curing process of polymer c is shown in Figure 6, and the demonstration of chromene formation by liquid-state 1H-NMR is shown in Figure 7.
In 1H-NMR, the presence of several well-characterized signals was noted. A methylene group appeared at 4.87 p.p.m. and a vinylidene group at 5.77 and 6.32 p.p.m., both characteristic of chromene.
Thermal stability of cured poly(dimethylsilylene-co-propynylene ether bisphenol)
The thermal stabilities of the cured polymers a, b and c were assessed by thermogravimetric analyses under nitrogen at a heating rate of 10 °C min−1. The thermograms of the three polymers are compiled in Figure 8, and the data are presented in Table 2. As shown in Figure 8, the decomposition process for the cured polymers was performed in one stage. The degradation temperatures at 5% weight loss (Td5) of the three cured polymers were above 400 °C and increased as follows: polymer c<polymer b<polymer a. The residue yields of polymers a, b and c at 800 °C were 69.0, 56.5 and 41.8%, respectively, whereas those at 1000 °C were 67.0, 55.2 and 40.5%, respectively. Thermal stability was dependent on the backbone structures and the cross-linking densities of the cured polymers. Structures of the polymers were mainly different in the bisphenol used. The thermal stability of isopropylidene group (in polymer b), resorcinol (in polymer a) and perfluoroisopropylidene group (in polymer c) increased in turn, but the steric hindrance of the perfluoroisopropylidene group was larger than that of the other two, which greatly reduced the cross-linking density of cured polymer c. The cross-linking density of cured polymer a was higher than that of polymers b and c; therefore, polymer a had the highest thermal stability of the three polymers.
Mechanical properties of carbon fiber-reinforced composites
Table 3 shows the properties of carbon fiber fabric (T300)-reinforced poly(dimethylsilylene-co-propynylene ether bisphenol) composites at room temperature. The flexural strength, flexural modulus and shear strength of polymer c composite were found to be 408 MPa, 53.3 GPa and 24.8 MPa, respectively. The composites of polymer c had better mechanical properties than did those of the other two, as shown in Table 3. The polymers became brittle with increasing cross-link density, and the embrittlement decreased the strength of the composites. The cross-linking density of cured polymer c was lower than that of polymers a and b. Therefore, the mechanical properties of polymer c composites were better than those of polymers a and b.
Conclusions
Three different poly(dimethylsilylene-co-propynylene ether bisphenol) resins were successfully synthesized. The structures and properties of the polymers were characterized by FT-IR, NMR, GPC, rheological analysis, DSC and thermogravimetric analysis. The processing windows of polymers a, b and c were 61–240 °C, 85–245 °C and 93–249 °C, respectively. The curing reaction took place at 240 °C for polymer a, 248 °C for polymer b and 258 °C for polymer c. The degradation temperatures of the three cured polymers at 5% weight loss (Td5) were all above 400 °C. Cured polymer a displayed the highest thermal stability, as derived from its cross-linking density. The flexural strength, flexural modulus and shear strength of composite of polymer c were 408 MPa, 53.3 GPa and 24.8 MPa, respectively. The composites of polymer c had better mechanical properties than did those of polymers a and b.
References
Narisawa, M., Tanaka, E., Nishimura, R., Okamura, K., Itoh, M. & Kamiyama, T. Synthesis and characterization of carbon-base hybrid ceramics in coating form from thermosetting resin-alkoxide mixtures. Key. Eng. Mater. 247, 137–140 (2003).
Narisawa, M., Endoh, Y., Tanaka, E., Nishimura, R., Mabuchi, H., Okamura, K., Itoh, M. & Kamiyama, T. Synthesis and nanostructure characterization of carbon base hybrid ceramics derived from Si–H containing resin–alkoxide mixtures. J. Phys. Chem. Solids 66, 565–570 (2005).
Zhang, J., Huang J., Zhou W., Huang F. & Du L. Fiber reinforced silicon-containing arylacetylene resin composites. Express. Polym. Lett. 1, 831–836 (2007).
Ogasawara, T., Ishikawa, T., Itoh, M., Abe, T., Yokota, R. & Ando, M. Carbon fiber reinforced composites with newly developed silicon containing polymer MSP. Adv. Composite Mater. 10, 319–327 (2001).
Itoh, M., Inoue, K., Hirayama, N., Sugimoto, M. & Seguchi, T. Fiber reinforced plastics using a new heat-resistant silicon based polymer. J. Mater. Sci. 37, 3795–3801 (2002).
Bouillon, E., Pailler, R. & Naslain, R. New poly(carbosi1ane) models. 5. Pyrolysis of a series of functional poly( carbosilanes). Chem. Mater. 3, 356–367 (1991).
Ijadi-Maghsoodi, S. & Barton, T. J. Synthesis and study of silylene-diacetylene polymers. Macromolecules 23, 4485–4486 (1990).
Narisawa, M., Hoshino, J. & Okamura, K. Synthesis of amorphous carbon fiber from a new organosilicon precursor. J. Mater. Sci. 35, 1535–1540 (2000).
Iwahara, T., Hayase, S. & West, R. Synthesis and properties of ethynylene-disilanylene copolymers. Macromolecules 23, 1298–1301 (1990).
Corriu, R. J. P., Guerin, C., Henner, B., Kuhlmann, T. & Alain, J. Organosilicon polymers: synthesis of poly[(silany1ene)diethynylene]s with conducting properties. Chem. Mater. 2, 351–352 (1990).
Ohshita, J., Iida, T., Ikeda, M., Uemura, T., Ohta, N. & Kuna, A. Synthesis of poly{[bis(diethynylphenyl)silylene]phenylene}s with highly heat-resistant properties and an application to conducting materials. J. Organomet. Chem. 689, 1540–1545 (2004).
Itoh, M., Mitsuzuka, M., Utsumi, T., Iwata, K. & Inoue, K. Dehydrogenative coupling reactions between hydrosilanes and monosubstituted alkynes catalyzed by solid bases. J. Organomet. Chem. 476, C30–C31 (1994).
Itoh, M., Mitsuzuka, M., Iwata, K. & Inoue, K. A novel synthesis and extremely high thermal stability of poly[(phenylsily1ene)-ethynylene-l,3-phenyleneethynylene]. Macromolecules 27, 7917–7919 (1994).
Itoh, M., Inoue, K., Iwata, K., Mitsuzuka, M. & Kakigano, T. New highly heat-resistant polymers containing silicon: poly(silyleneethynylenephenyleneethynylene)s. Macromolecules 30, 694–701 (1997).
Itoh, M. A novel synthesis of a highly heat-resistant organosilicon polymer using base catalysts. Catal. Surv. Jpn 3, 61–69 (1999).
Buvat, P., Levassort, C. & Jousse, F. Poly(silyleneetheynylene phenyleneethynylene) and methods for preparing same. W. O. Pat. 019899, 1–49 (2001).
Levassort, C., Jousse, F., Delnaud, L. & Buvat, P. Poly(etheynylene phenylene ethynylene silylene) comprising an inert spacer and methods for preparing same. W.O. Pat. 038652, 1–61 (2002).
Wang, F., Zhang, J., Huang, J., Yan, H., Huang, F. & Du, L. Synthesis and characterization of poly(dimethylsilylene ethynylenephenyleneethynylene) terminated with phenylacetylene. Polym. Bull. 56, 19–26 (2006).
Inbasekaran, M. N., Dirlikov, S. K. & Psilanti, Y. Process for making propargyl ethers of bisphenols. US Pat. 4885403, 1–18 (1989).
Dirlikov, S. K. Propargyl-terminated resins—a hydrophobic substitute for epoxy resins. High. Penform. Polym. 2, 67–77 (1990).
Douglas, W. E. & Overend, A. S. Curing reactions in acetylene terminated resins–I. Uncatalyzed cure of arylpropargyl ether terminated monomers. Eur. Polym. J. 27, 1279–1287 (1991).
Douglas, W. E. & Overend, A. S. Cyclotrimerization versus non-aromatic polyene formation in catalysed cure of an arylpropargyl-ether-terminated monomer. Polymer 34, 1544–1545 (1993).
Grenier-Loustalot, M. F. & Sanglar, C. Prepolymers with propargylic terminal residues—I. Simulation of reaction mechanisms and kinetics on monofunctional models. Eur. Polym. J. 33, 1125–1134 (1997).
Reghunadhan Nair, C. P., Bindu, R. L., Krishnan, K. & Ninan., K. N. Bis propargyl ether resins: synthesis and structure-thermal property correlations. Eur. Polym. J. 35, 235–246 (1999).
Liu, F., Li, W., Wei, L. & Zhao, T. Bismaleimide modified bis propargyl ether bisphenol A resin: synthesis, cure, and thermal properties. J. Appl. Polym. Sci. 102, 3610–3615 (2006).
Wang, F., Xu, J., Zhang, J., Huang, F., Shen, Y. & Du, L. Synthesis and thermal cure of diphenyl ethers terminated with acetylene and phenylacetylene. Polym. Int. 55, 1063–1068 (2006).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, F., Wang, C., Shen, X. et al. Synthesis and characterization of novel silicon-containing aromatic bispropargyl ether resins and their composites. Polym J 43, 594–599 (2011). https://doi.org/10.1038/pj.2011.39
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/pj.2011.39
Keywords
This article is cited by
-
Enhance high-temperature mechanical performance of a silicon-containing arylether arylacetylene resin with the aid of a terminal alkyne compound
Journal of Polymer Research (2021)
-
Synthesis and Properties of Polymers with an Organosilicon–Acetylene Backbone
Journal of Inorganic and Organometallic Polymers and Materials (2018)