Abstract
Background:
The Prostate Cancer Prevention Trial Risk Calculator 2.0 (PCPTRC) is a widely used risk-based calculator used to assess a man’s risk of prostate cancer (PCa) before biopsy. This risk calculator was created from data of a patient cohort undergoing a 6-core sextant biopsy, and subsequently validated in men undergoing 12-core systematic biopsy (SBx). The accuracy of the PCPTRC has not been studied in patients undergoing magnetic resonance imaging/ultrasound (MRI/US) fusion-guided biopsy (FBx). We sought to assess the performance of the PCPTRC for straitifying PCa risk in a FBx cohort.
Methods:
A review of a prospective cohort undergoing MRI and FBx/SBx was conducted. Data from consecutive FBx/SBx were collected between August 2007 and February 2014, and PCPTRC scores using the PCPTRC2.0R-code were calculated. The risk of positive biopsy and high-grade cancer (Gleason ⩾7) on biopsy was calculated and compared with overall and high-grade cancer detection rates (CDRs). Receiver operating characteristic curves were generated and the areas under the curves (AUCs) were compared using DeLong’s test.
Results:
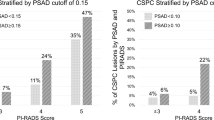
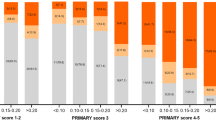
Of 595 men included in the study, PCa was detected in 39% (232) by SBx compared with 48% (287) on combined FBx/SBx biopsy. The PCPTRC AUCs for the CDR were similar (P=0.70) for SBx (0.69) and combined biopsy (0.70). For high-grade disease, AUCs for SBx (0.71) and combined biopsy (0.70) were slightly higher, but were not statistically different (P=0.55).
Conclusions:
In an MRI-screened population of men undergoing FBx, PCPTRC continues to represent a practical method of accurately stratifying PCa risk.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 4 print issues and online access
$259.00 per year
only $64.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Siddiqui MM, Rais-Bahrami S, Turkbey B, George AK, Rothwax J, Shakir N et al. Comparison of MR/ultrasound fusion-guided biopsy with ultrasound-guided biopsy for the diagnosis of prostate cancer. JAMA 2015; 313: 390–397.
Filson CP, Natarajan S, Margolis DJ, Huang J, Lieu P, Dorey FJ et al. Prostate cancer detection with magnetic resonance-ultrasound fusion biopsy: the role of systematic and targeted biopsies. Cancer 2016; 122: 884–892.
Meigs JB, Barry MJ, Oesterling JE, Jacobsen SJ . Interpreting results of prostate-specific antigen testing for early detection of prostate cancer. J Gen Intern Med 1996; 11: 505–512.
Barry MJ . Clinical practice. Prostate-specific-antigen testing for early diagnosis of prostate cancer. N Engl J Med 2001; 344: 1373–1377.
Loeb S, Bjurlin MA, Nicholson J, Tammela TL, Penson DF, Carter HB et al. Overdiagnosis and overtreatment of prostate cancer. Eur Urol 2014; 65: 1046–1055.
Thompson IM, Goodman PJ, Tangen CM, Lucia MS, Miller GJ, Ford LG et al. The influence of finasteride on the development of prostate cancer. N Engl J Med 2003; 349: 215–224.
Thompson IM, Ankerst DP, Chi C, Goodman PJ, Tangen CM, Lucia MS et al. Assessing prostate cancer risk: results from the Prostate Cancer Prevention Trial. J Natl Cancer Inst 2006; 98: 529–534.
Ankerst DP, Hoefler J, Bock S, Goodman PJ, Vickers A, Hernandez J et al. Prostate Cancer Prevention Trial risk calculator 2.0 for the prediction of low- vs high-grade prostate cancer. Urology 2014; 83: 1362–1367.
Lundon DJ, Kelly BD, Foley R, Loeb S, Fitzpatrick JM, Watson RW et al. Prostate cancer risk assessment tools in an unscreened population. World J Urol 2015; 33: 827–832.
Oliveira M, Marques V, Carvalho AP, Santos A . Head-to-head comparison of two online nomograms for prostate biopsy outcome prediction. BJU Int 2011; 107: 1780–1783.
Trottier G, Roobol MJ, Lawrentschuk N, Bostrom PJ, Fernandes KA, Finelli A et al. Comparison of risk calculators from the Prostate Cancer Prevention Trial and the European Randomized Study of Screening for Prostate Cancer in a contemporary Canadian cohort. BJU Int 2011; 108: E237–E244.
Poyet C, Nieboer D, Bhindi B, Kulkarni GS, Wiederkehr C, Wettstein MS et al. Prostate cancer risk prediction using the novel versions of the European Randomised Study for Screening of Prostate Cancer (ERSPC) and Prostate Cancer Prevention Trial (PCPT) risk calculators: independent validation and comparison in a contemporary European cohort. BJU Int 2016; 117: 401–408.
Foley RW, Maweni RM, Gorman L, Murphy K, Lundon DJ, Durkan G et al. The ERSPC risk calculators significantly outperform the PCPT 2.0 in the prediction of prostate cancer; a multi-institutional study. BJU Int 2016; 118: 706–714.
Ploussard G, Epstein JI, Montironi R, Carroll PR, Wirth M, Grimm MO et al. The contemporary concept of significant versus insignificant prostate cancer. Eur Urol 2011; 60: 291–303.
Ankerst DP, Boeck A, Freedland SJ, Jones JS, Cronin AM, Roobol MJ et al. Evaluating the Prostate Cancer Prevention Trial High Grade Prostate Cancer Risk Calculator in 10 international biopsy cohorts: results from the Prostate Biopsy Collaborative Group. W J Urol 2014; 32: 185–191.
Nam RK, Kattan MW, Chin JL, Trachtenberg J, Singal R, Rendon R et al. Prospective multi-institutional study evaluating the performance of prostate cancer risk calculators. J Clini Oncol 2011; 29: 2959–2964.
Siddiqui MM, George AK, Rubin R, Rais-Bahrami S, Parnes HL, Merino MJ et al. Efficiency of Prostate Cancer Diagnosis by MR/Ultrasound Fusion-Guided Biopsy vs Standard Extended-Sextant Biopsy for MR-Visible Lesions. J Natl Cancer Inst 2016; 108: djw039.
Rais-Bahrami S, Siddiqui MM, Turkbey B, Stamatakis L, Logan J, Hoang AN et al. Utility of multiparametric magnetic resonance imaging suspicion levels for detecting prostate cancer. J Urol 2013; 190: 1721–1727.
Mertan FV, Greer MD, Shih JH, George AK, Kongnyuy M, Muthigi A et al. Prospective Evaluation of the Prostate Imaging Reporting and Data System Version 2 for Prostate Cancer Detection. J Urol 2016; S0022-S5347: 30199–30199.
Muller BG, Shih JH, Sankineni S, Marko J, Rais-Bahrami S, George AK et al. Prostate cancer: interobserver agreement and accuracy with the revised prostate imaging reporting and data system at multiparametric MR imaging. Radiology 2015; 277: 741–750.
Ankerst DP, Till C, Boeck A, Goodman P, Tangen CM, Feng Z et al. The impact of prostate volume, number of biopsy cores and american urological association symptom score on the sensitivity of cancer detection using the Prostate Cancer Prevention Trial Risk Calculator. J Urol 2013; 190: 70–76.
Eyre SJ, Ankerst DP, Wei JT, Nair PV, Regan MM, Bueti G et al. Validation in a multiple urology practice cohort of the Prostate Cancer Prevention Trial calculator for predicting prostate cancer detection. J Urol 2009; 182: 2653–2658.
Sankineni S, George AK, Brown AM, Rais-Bahrami S, Wood BJ, Merino MJ et al. Posterior subcapsular prostate cancer: identification with mpMRI and MRI/TRUS fusion-guided biopsy. Abdom Imaging 2015; 40: 2557–2565.
Volkin D, Turkbey B, Hoang AN, Rais-Bahrami S, Yerram N, Walton-Diaz A et al. Multiparametric magnetic resonance imaging (MRI) and subsequent MRI/ultrasonography fusion-guided biopsy increase the detection of anteriorly located prostate cancers. BJU Int 2014; 114: E43–E49.
Salami SS, Vira MA, Turkbey B, Fakhoury M, Yaskiv O, Villani R et al. Multiparametric magnetic resonance imaging outperforms the Prostate Cancer Prevention Trial risk calculator in predicting clinically significant prostate cancer. Cancer 2014; 120: 2876–2882.
Esen T, Turkbey B, Patel A, Futterer J . Multiparametric MRI in prostate cancer. BioMed Res Int 2014; 2014: 296810.
Sonn GA, Chang E, Natarajan S, Margolis DJ, Macairan M, Lieu P et al. Value of targeted prostate biopsy using magnetic resonance-ultrasound fusion in men with prior negative biopsy and elevated prostate-specific antigen. Euro Urol 2014; 65: 809–815.
DeLong ER, DeLong DM, Clarke-Pearson DL . Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 1988; 44: 837–845.
Acknowledgements
This research was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Competing interests
NIH and Philips Healthcare have a cooperative research and development agreement. NIH and Philips share intellectual property in the field.
Additional information
DISCLOSURE
NIH and Philips Healthcare have a cooperative research and development agreement. NIH and Philips share intellectual property in the field.
Rights and permissions
About this article
Cite this article
Maruf, M., Fascelli, M., George, A. et al. The prostate cancer prevention trial risk calculator 2.0 performs equally for standard biopsy and MRI/US fusion-guided biopsy. Prostate Cancer Prostatic Dis 20, 179–185 (2017). https://doi.org/10.1038/pcan.2016.46
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/pcan.2016.46