Abstract
Background:
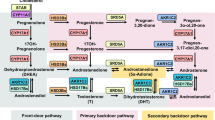
Epidemiologic and in vitro studies suggest that SLCO-encoded organic anion transporting polypeptide (OATP) transporters influence the response of prostate cancer (PCa) to androgen deprivation by altering intratumor androgens. We have previously shown that castration-resistant metastases express multiple SLCO transporters at significantly higher levels than primary PCa, suggesting that OATP-mediated steroid transport is biologically relevant in advanced disease. However, whether OATP-mediated steroid transport can actually modify prostate tumor androgen levels in vivo has never been demonstrated.
Methods:
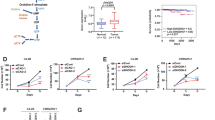
We sought to determine whether OATP-mediated steroid transport can measurably alter PCa androgen levels in vivo. We evaluated the uptake of dehydroepiandrosterone (DHEAS), E1S and testosterone in LNCaP cells engineered to express OATP1B1, 1B3, 2B1 or 4A1. We measured the uptake via administration of tritiated steroids to castrate mice bearing vector control or OATP1B1-, 2B1- or 4A1-expressing xenografts. We treated tumor-bearing mice with DHEAS and testosterone at physiologically relevant levels and measured intratumor accumulation of administered steroids by mass spectrometry.
Results:
OATP1B1- and 2B-expressing xenografts each showed a threefold increase in tritiated-DHEAS uptake vs vector controls (P=0.002 and P=0.036, respectively). At circulating DHEAS levels similar to those in abiraterone-treated men (~15 μg dl−1), OATP1B1- and 2B1-expressing xenografts showed a 3.9-fold (P=0.057) and 1.9-fold (P=0.048) increase in tumor accumulation of DHEAS and a 1.6-fold (P=0.057) and 2.7-fold (P=0.095) increase in DHEA, respectively. At the substantial circulating testosterone levels found in eugonadal men, a consistent effect of OATP1B1, 2B1 or 4A1 on testosterone uptake in vivo was not detected.
Conclusions:
OATP transporters measurably alter DHEAS uptake and intratumor androgen levels in prostate tumors in vivo, even at circulating androgen levels achieved in abiraterone-treated patients. These novel data emphasize the continued need to inhibit ligand-mediated androgen receptor signaling in PCa tumors, and support prospective evaluation of studies designed to test inhibition of OATP-mediated DHEAS uptake and utilization.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 4 print issues and online access
$259.00 per year
only $64.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Nelson PS, Clegg N, Arnold H, Ferguson C, Bonham M, White J et al. The program of androgen-responsive genes in neoplastic prostate epithelium. Proc Natl Acad Sci USA 2002; 99: 11890–11895.
Scher HI, Buchanan G, Gerald W, Butler LM, Tilley WD . Targeting the androgen receptor: improving outcomes for castration-resistant prostate cancer. Endocr Relat Cancer 2004; 11: 459–476.
Mohler JL, Gregory CW, Ford OH III, Kim D, Weaver CM, Petrusz P et al. The androgen axis in recurrent prostate cancer. Clin Cancer Res 2004; 10: 440–448.
Mostaghel EA, Page ST, Lin DW, Fazli L, Coleman IM, True LD et al. Intraprostatic androgens and androgen-regulated gene expression persist after testosterone suppression: therapeutic implications for castration-resistant prostate cancer. Cancer Res 2007; 67: 5033–5041.
Montgomery RB, Mostaghel EA, Vessella R, Hess DL, Kalhorn TF, Higano CS et al. Maintenance of intratumoral androgens in metastatic prostate cancer: a mechanism for castration-resistant tumor growth. Cancer Res 2008; 68: 4447–4454.
Stanbrough M, Bubley GJ, Ross K, Golub TR, Rubin MA, Penning TM et al. Increased expression of genes converting adrenal androgens to testosterone in androgen-independent prostate cancer. Cancer Res 2006; 66: 2815–2825.
Taplin ME, Montgomery B, Logothetis CJ, Bubley GJ, Richie JP, Dalkin BL et al. Intense androgen-deprivation therapy with abiraterone acetate plus leuprolide acetate in patients with localized high-risk prostate cancer: results of a Randomized Phase II Neoadjuvant Study. J Clin Oncol 2014; 32: 3705–3715.
Tamae D, Mostaghel E, Montgomery B, Nelson PS, Balk SP, Kantoff PW et al. The DHEA-sulfate depot following P450c17 inhibition supports the case for AKR1C3 inhibition in high risk localized and advanced castration resistant prostate cancer. Chemico-biol Interact 2015; 234: 332–338.
Hagenbuch B, Meier PJ . The superfamily of organic anion transporting polypeptides. Biochim Biophys Acta 2003; 1609: 1–18.
Pizzagalli F, Varga Z, Huber RD, Folkers G, Meier PJ, St-Pierre MV . Identification of steroid sulfate transport processes in the human mammary gland. J Clin Endocrinol Metab 2003; 88: 3902–3912.
Tamai I, Nezu J, Uchino H, Sai Y, Oku A, Shimane M et al. Molecular identification and characterization of novel members of the human organic anion transporter (OATP) family. Biochem Biophys Res Commun 2000; 273: 251–260.
Ugele B, St-Pierre MV, Pihusch M, Bahn A, Hantschmann P . Characterization and identification of steroid sulfate transporters of human placenta. Am J Physiol Endocrinol Metab 2003; 284: E390–E398.
Juang HH, Lin YF, Chang PL, Tsui KH . Cardiac glycosides decrease prostate specific antigen expression by down-regulation of prostate derived Ets factor. J Urol 2010; 184: 2158–2164.
Lin J, Denmeade S, Carducci MA . HIF-1alpha and calcium signaling as targets for treatment of prostate cancer by cardiac glycosides. Curr Cancer Drug Targets 2009; 9: 881–887.
Clements A, Gao B, Yeap SH, Wong MK, Ali SS, Gurney H . Metformin in prostate cancer: two for the price of one. Ann Oncol 2011; 22: 2556–2560.
Zhang Y, Hays A, Noblett A, Thapa M, Hua DH, Hagenbuch B . Transport by OATP1B1 and OATP1B3 enhances the cytotoxicity of epigallocatechin 3-O-gallate and several quercetin derivatives. J Nat Prod 2013; 76: 368–373.
Moses MA, Henry EC, Ricke WA, Gasiewicz TA . The heat shock protein 90 inhibitor, (−)-epigallocatechin gallate, has anticancer activity in a novel human prostate cancer progression model. Cancer Prev Res (Phila) 2015; 8: 249–257.
Yang M, Xie W, Mostaghel E, Nakabayashi M, Werner L, Sun T et al. SLCO2B1 and SLCO1B3 may determine time to progression for patients receiving androgen deprivation therapy for prostate cancer. J Clin Oncol 2011; 29: 2565–2573.
Arakawa H, Nakanishi T, Yanagihara C, Nishimoto T, Wakayama T, Mizokami A et al. Enhanced expression of organic anion transporting polypeptides (OATPs) in androgen receptor-positive prostate cancer cells: possible role of OATP1A2 in adaptive cell growth under androgen-depleted conditions. Biochem Pharmacol 2012; 84: 1070–1077.
Wright JL, Kwon EM, Ostrander EA, Montgomery B, Line DW, Vessella RL et al. Expression of SLCO transport genes in castration resistant prostate cancer and impact of genetic variation in SCLO1B3 and SLCO2B1 on prostate cancer outcomes. Cancer Epidemiol Biomarkers Prev 2011; 20: 619–627.
Cho E, Mostaghel EA, Russell KJ, Liao JJ, Konodi MA, Kurland BF et al. External beam radiation therapy and abiraterone in men with localized prostate cancer: safety and effect on tissue androgens. Int J Radiat Oncol Biol Phys 2015; 92: 236–243.
Konig J, Cui Y, Nies AT, Keppler D . A novel human organic anion transporting polypeptide localized to the basolateral hepatocyte membrane. Am J Physiol Gastrointest Liver Physiol 2000; 278: G156–G164.
Konig J, Cui Y, Nies AT, Keppler D . Localization and genomic organization of a new hepatocellular organic anion transporting polypeptide. J Biol Chem 2000; 275: 23161–23168.
Tschantz WR, Pfeifer ND, Meade CL, Wang L, Lanzetti A, Kamath AV et al. Expression, purification and characterization of the human membrane transporter protein OATP2B1 from Sf9 insect cells. Protein Expr Purif 2008; 57: 163–171.
Hagenbuch B, Meier PJ . Organic anion transporting polypeptides of the OATP/ SLC21 family: phylogenetic classification as OATP/SLCO superfamily, new nomenclature and molecular/functional properties. Pflugers Arch 2004; 447: 653–665.
Baldes C, Koenig P, Neumann D, Lenhof HP, Kohlbacher O, Lehr CM . Development of a fluorescence-based assay for screening of modulators of human organic anion transporter 1B3 (OATP1B3). Eur J Pharma Biopharma 2006; 62: 39–43.
Mueller JW, Gilligan LC, Idkowiak J, Arlt W, Foster PA . The regulation of steroid action by sulfation and desulfation. Endocr Rev 2015; 36: 526–563.
Hagenbuch B, Stieger B . The SLCO (former SLC21) superfamily of transporters. Mol Aspects Med 2013; 34: 396–412.
Hamada A, Sissung T, Price DK, Danesi R, Chau CH, Sharifi N et al. Effect of SLCO1B3 haplotype on testosterone transport and clinical outcome in caucasian patients with androgen-independent prostatic cancer. Clin Cancer Res 2008; 14: 3312–3318.
Kalliokoski A, Niemi M . Impact of OATP transporters on pharmacokinetics. Br J Pharmacol 2009; 158: 693–705.
Obaidat A, Roth M, Hagenbuch B . The expression and function of organic anion transporting polypeptides in normal tissues and in cancer. Annu Rev Pharmacol Toxicol 2012; 52: 135–151.
Kullak-Ublick GA, Ismair MG, Stieger B, Landmann L, Huber R, Pizzagalli F et al. Organic anion-transporting polypeptide B (OATP-B) and its functional comparison with three other OATPs of human liver. Gastroenterology 2001; 120: 525–533.
Maeda T, Irokawa M, Arakawa H, Kuraoka E, Nozawa T, Tateoka R et al. Uptake transporter organic anion transporting polypeptide 1B3 contributes to the growth of estrogen-dependent breast cancer. J Steroid Biochem Mol Biol 2010; 122: 180–185.
Cui Y, Konig J, Leier I, Buchholz U, Keppler D . Hepatic uptake of bilirubin and its conjugates by the human organic anion transporter SLC21A6. J Biol Chem 2001; 276: 9626–9630.
Koenen A, Kock K, Keiser M, Siegmund W, Kroemer HK, Grube M . Steroid hormones specifically modify the activity of organic anion transporting polypeptides. Eur J Pharm Sci 2012; 47: 774–780.
Grube M, Kock K, Karner S, Reuther S, Ritter CA, Jedlitschky G et al. Modification of OATP2B1-mediated transport by steroid hormones. Mol Pharmacol 2006; 70: 1735–1741.
Leon CG, Locke JA, Adomat HH, Etinger SL, Twiddy AL, Neumann RD et al. Alterations in cholesterol regulation contribute to the production of intratumoral androgens during progression to castration-resistant prostate cancer in a mouse xenograft model. Prostate 2009; 70: 390–400.
Selcer KW, Kabler H, Sarap J, Xiao Z, Li PK . Inhibition of steryl sulfatase activity in LNCaP human prostate cancer cells. Steroids 2002; 67: 821–826.
Mostaghel EA . Steroid hormone synthetic pathways in prostate cancer. Transl Androl Urol 2013; 2: 212–227.
Ryan CJ, Halabi S, Ou SS, Vogelzang NJ, Kantoff P, Small EJ . Adrenal androgen levels as predictors of outcome in prostate cancer patients treated with ketoconazole plus antiandrogen withdrawal: results from a cancer and leukemia group B study. Clin Cancer Res 2007; 13: 2030–2037.
Kim W, Zhang L, Wilton JH, Fetterly G, Mohler JL, Weinberg V et al. Sequential use of the androgen synthesis inhibitors ketoconazole and abiraterone acetate in castration-resistant prostate cancer and the predictive value of circulating androgens. Clin Cancer Res 2014; 20: 6269–6276.
Sharifi N, Hamada A, Sissung T, Danesi R, Venzon D, Baum C et al. A polymorphism in a transporter of testosterone is a determinant of androgen independence in prostate cancer. BJU Int 2008; 102: 617–621.
Al Sarakbi W, Mokbel R, Salhab M, Jiang WG, Reed MJ, Mokbel K . The role of STS and OATP-B mRNA expression in predicting the clinical outcome in human breast cancer. Anticancer Res 2006; 26: 4985–4990.
Nozawa T, Suzuki M, Yabuuchi H, Irokawa M, Tsuji A, Tamai I . Suppression of cell proliferation by inhibition of estrone-3-sulfate transporter in estrogen-dependent breast cancer cells. Pharm Res 2005; 22: 1634–1641.
Bosland MC . A perspective on the role of estrogen in hormone-induced prostate carcinogenesis. Cancer Lett 2013; 334: 28–33.
Giton F, de la Taille A, Allory Y, Galons H, Vacherot F, Soyeux P et al. Estrone sulfate (E1S), a prognosis marker for tumor aggressiveness in prostate cancer (PCa). J Steroid Biochem Mol Biol 2008; 109: 158–167.
Giton F, Sirab N, Franck G, Gervais M, Schmidlin F, Ali T et al. Evidence of estrone-sulfate uptake modification in young and middle-aged rat prostate. J Steroid Biochem Mol Biol 2015; 152: 89–100.
Harshman LC, Wang X, Nakabayashi M, Xie W, Valenca L, Werner L et al. Statin use at the time of initiation of androgen deprivation therapy and time to progression in patients with hormone-sensitive prostate cancer. JAMA Oncol 2015; 1: 495–504.
Purohit A, Foster PA . Steroid sulfatase inhibitors for estrogen- and androgen-dependent cancers. J Endocrinol 2012; 212: 99–110.
Terman JR, Kashina A . Post-translational modification and regulation of actin. Curr Opin Cell Biol 2013; 25: 30–38.
Yao J, Hong W, Huang J, Zhan K, Huang H, Hong M . N-glycosylation dictates proper processing of organic anion transporting polypeptide 1B1. PLoS One 2012; 7: e52563.
Acknowledgements
We acknowledge the expert technical assistance provided by Steve Mongovin, Brandy Olin, Bea Binang and Dominic Tran. This work was supported by Pacific Northwest Prostate Cancer SPORE P50 CA97186 (to EAM); NIH/NCI Cancer Center Support Grant 5P30CA015704-40 (to EAM, AK) and Department of Defense CDMRP W81XWH-11-2-0154 (to EAM).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Prostate Cancer and Prostatic Diseases website
Supplementary information
Rights and permissions
About this article
Cite this article
Green, S., Kaipainen, A., Bullock, K. et al. Role of OATP transporters in steroid uptake by prostate cancer cells in vivo. Prostate Cancer Prostatic Dis 20, 20–27 (2017). https://doi.org/10.1038/pcan.2016.42
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/pcan.2016.42
This article is cited by
-
Effects of the SLCO1B1 A388G single nucleotide polymorphism on the development, clinical parameters, treatment, and survival of multiple myeloma cases in a Polish population
Molecular Biology Reports (2023)
-
Androgen receptor signalling impairs docetaxel efficacy in castration-resistant prostate cancer
British Journal of Cancer (2020)
-
Association of prostate cancer SLCO gene expression with Gleason grade and alterations following androgen deprivation therapy
Prostate Cancer and Prostatic Diseases (2019)