Abstract
Background:
Magnetic resonance imaging (MRI) is not routinely performed before initiating radium-223 to document spinal epidural disease. However, radium-223 decays to form α-particles with very short path lengths that may not reach the epidural space. Herein, we investigate the impact of baseline spinal epidural disease on metastatic castration-resistant prostate cancer (mCRPC) patients treated with radium-223.
Methods:
Between October 2013 to December 2014, 41 consecutive mCRPC patients at a large tertiary cancer center were prescribed radium-223 as part of standard of care. 29% of patients had pre-treatment epidural disease (posMRI), 27% had no epidural disease (negMRI), and 44% did not have a baseline MRI (noMRI). All patients had post-treatment spinal imaging. Actuarial survival times were calculated for overall survival (OS), spinal axis radiographic progression-free survival (spinePFS) and epidural progression-free survival (epiPFS) from time of first radium-223 treatment.
Results:
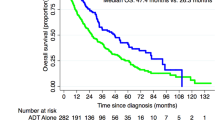
For patients with posMRI (n=12), noMRI (n=18) and negMRI (n=11) cumulative rates of development or worsening of epidural disease and/or high-grade cord compression at time of last follow-up were 83%, 44% and 9%, respectively (P=0.001). For the posMRI, noMRI and negMRI groups the median OS was 6.3 months, 12.6 months and not reached (P=0.01), the median spinePFS was 3.2 months, 4.8 months and not reached (P=0.01), and the median epiPFS was 3.2 months, 10.4 months and not reached (P=0.001). Completing less than six cycles of radium-223 was significantly associated with worse OS (P<0.0001), spinePFS (P=0.007) and epiPFS (P=0.01). Greater than or equal to twenty osseous lesions pre-treatment was significantly associated with worse spinePFS (P=0.001) and epiPFS (P=0.03).
Conclusions:
In a heavily pre-treated small cohort, patients with baseline epidural disease frequently progressed to spinal cord compression and early cessation of radium-223 therapy. Studies are needed to determine the optimal timing of radium-223 with other mCRPC therapies given the predilection for epidural disease and treatment failure after multiple prior lines of mCRPC therapy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 4 print issues and online access
$259.00 per year
only $64.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Parker C, Nilsson S, Heinrich D, Helle SI, O'Sullivan JM, Fosså SD . et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med 2013; 369: 213–223.
Cabot RC, Harris NL, Rosenberg ES, Shepard J-AO, Cort AM, Ebeling SH et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med 2012; 367: 1187–1197.
de Bono JS, Oudard S, Ozguroglu M, Hansen S, Machiels J-P, Kocak I et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet 2010; 376: 1147–1154.
De Bono JS, Logothetis CJ, Molina A, Fizazi K, North S, Chu L et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med 2011; 364: 1995–2005.
Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med 2010; 363: 411–422.
Gillessen S, Omlin A, Attard G, de Bono J, Efstathiou E, Fizazi K et al. Management of patients with advanced prostate cancer: recommendations of the St Gallen Advanced Prostate Cancer Consensus Conference (APCCC) 2015. Ann Oncol 2015; 26: 1589–1604.
Mohler J, Armstrong A, Bahnson R . NCCN clinical practice guidelines for prostate cancer. J Natl Compr Canc Netw 2015; 8: 162–200.
Buhmann S, Becker C, Duerr HR, Reiser M, Baur-Melnyk A . Detection of osseous metastases of the spine: comparison of high resolution multi-detector-CT with MRI. Eur J Radiol 2009; 69: 567–573.
Sartor O, Reid RH, Hoskin PJ, Quick DP, Ell PJ, Coleman RE et al. Samarium-153-Lexidronam complex for treatment of painful bone metastases in hormone-refractory prostate cancer. Urology 2004; 63: 940–945.
Bubendorf L, Schöpfer A, Wagner U, Sauter G, Moch H, Willi N et al. Metastatic patterns of prostate cancer: an autopsy study of 1,589 patients. Hum Pathol 2000; 31: 578–583.
Janjan NA . Radiation for bone metastases. Cancer 1997; 80: 1628–1645.
Lutz S, Berk L, Chang E, Chow E, Hahn C, Hoskin P et al. Palliative radiotherapy for bone metastases: an ASTRO evidence-based guideline. Int J Radiat Oncol Biol Phys 2011; 79: 965–976.
Gunawardana DH, Lichtenstein M, Better N, Rosenthal M . Results of strontium-89 therapy in patients with prostate cancer resistant to chemotherapy. Clin Nuclear Med 2004; 29: 81–85.
Zelefsky MJ, Scher HI, Forman JD, Linares LA, Curley T, Fuks Z . Palliative hemiskeletal irradiation for widespread metastatic prostate cancer: a comparison of single dose and fractionated regimens. Int J Radiat Oncol Biol Phys 1989; 17: 1281–1285.
Hoskin P, Sartor O, O'Sullivan JM, Johannessen DC, Helle SI, Logue J et al. Efficacy and safety of radium-223 dichloride in patients with castration-resistant prostate cancer and symptomatic bone metastases, with or without previous docetaxel use: a prespecified subgroup analysis from the randomised, double-blind, phase 3 ALSYMPCA trial. Lancet Oncol 2014; 15: 1397–1406.
Polkinghorn WR, Parker JS, Lee MX, Kass EM, Spratt DE, Iaquinta PJ et al. Androgen receptor signaling regulates DNA repair in prostate cancers. Cancer Discov 2013; 3: 1245–1253.
Spratt DE, Evans MJ, Davis BJ, Doran MG, Lee MX, Shah N et al. Androgen receptor upregulation mediates radioresistance after ionizing radiation. Cancer Res 2015; 75: 4688–4696.
Acknowledgements
Supported by the Prostate Cancer Foundation Rebecca and Nathan Milikowsky Young Investigator Award (DES). MJM and JRO have received funding and are conducting a clinical trial with Bayer.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The other authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Prostate Cancer and Prostatic Diseases website
Supplementary information
Rights and permissions
About this article
Cite this article
Spratt, D., Osborne, J., Zumsteg, Z. et al. Radium-223 outcomes after multiple lines of metastatic castration-resistant prostate cancer therapy in clinical practice: implication of pre-treatment spinal epidural disease. Prostate Cancer Prostatic Dis 19, 271–276 (2016). https://doi.org/10.1038/pcan.2016.14
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/pcan.2016.14
This article is cited by
-
Radiotheranostics in advanced prostate cancer: Current and future directions
Prostate Cancer and Prostatic Diseases (2024)