Abstract
Background:
This study was initiated to explore the impact of organic cation transporter 1 (OCT1) and multidrug and toxin extrusion transporter 1 (MATE1) genetic polymorphisms on toxicity, and clinical activity of metformin in patients with castration-resistant prostate cancer (CRPC).
Methods:
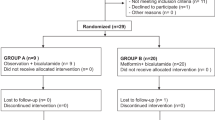
The SAKK 08/09 trial included 44 patients with CRPC to receive single-agent metformin 1000 mg two times a day until disease progression or unwanted toxicity. Drug pathway-associated gene polymorphisms of OCT1 (rs622342) and MATE1 (rs2289669) were assessed. The primary objective of this study was to define the relationship between mutations in OCT1, MATE1 and progression-free survival (PFS) at 12 weeks absolute PFS and PSA response in consenting patients of SAKK 08/09. The secondary objective of this study was to analyze the association between mutations in OCT1, MATE1, metformin-related toxicity, PSA response at 12 weeks and overall survival.
Results:
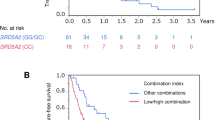
Thirty-six patients were evaluable for pharmacogenetic analysis. Homozygous carriers of the polymorphic OCT1 C-allele had no metformin-related toxicity as compared with 41.9% for any metformin-related toxicity in carriers of at least one wild-type A-allele (P=0.07). Disease progression according to RECIST (Response Evaluation Criteria In Solid Tumors) was significantly more frequent in homozygous carriers of the polymorphic OCT1 C-allele (80%) as compared with carriers of at least one wild-type A-allele (28.6%) (P=0.002). Disease progression according to RECIST was also more frequent in carriers of at least one polymorphic MATE1 A-allele (44%) as compared with homozygous carriers of the wild-type G-allele (12.5%) (P=0.07). OCT1 and MATE1 were not associated with PFS.
Conclusions:
The polymorphic OCT1 C-allele has been shown to be associated with less metformin-related toxicity and a higher risk of tumor progression in patients with CRPC receiving metformin as an anticancer treatment. Polymorphisms in metformin drug transporters are attractive molecular markers to serve as potential predictors of efficacy in future clinical studies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 4 print issues and online access
$259.00 per year
only $64.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Evans JM, Donnelly LA, Emslie-Smith AM, Alessi DR, Morris AD . Metformin and reduced risk of cancer in diabetic patients. BMJ 2005; 330: 1304–1305.
Preston MA, Riis AH, Ehrenstein V, Breau RH, Batista JL, Olumi AF et al. Metformin use and prostate cancer risk. Eur Urol 2014; 66: 1012–1020.
Ben Sahra I, Regazzetti C, Robert G, Laurent K, Le Marchand-Brustel Y, Auberger P et al. Metformin, independent of AMPK, induces mTOR inhibition and cell-cycle arrest through REDD1. Cancer Res 2011; 71: 4366–4372.
Ben Sahra I, Laurent K, Giuliano S, Larbret F, Ponzio G, Gounon P et al. Targeting cancer cell metabolism: the combination of metformin and 2-deoxyglucose induces p53-dependent apoptosis in prostate cancer cells. Cancer Res 2010; 70: 2465–2475.
Colquhoun AJ, Venier NA, Vandersluis AD, Besla R, Sugar LM, Kiss A et al. Metformin enhances the antiproliferative and apoptotic effect of bicalutamide in prostate cancer. Prostate Cancer Prostatic Dis 2012; 15: 346–352.
Margel D, Urbach DR, Lipscombe LL, Bell CM, Kulkarni G, Austin PC et al. Metformin use and all-cause and prostate cancer-specific mortality among men with diabetes. J Clin Oncol 2013; 31: 3069–3075.
Bensimon L, Yin H, Suissa S, Pollak MN, Azoulay L . The use of metformin in patients with prostate cancer and the risk of death. Cancer Epidemiol Biomarkers Prev 2014; 23: 2111–2118.
Andrzejewski S, Gravel SP, Pollak M, St-Pierre J . Metformin directly acts on mitochondria to alter cellular bioenergetics. Cancer Metab 2014; 2: 12.
Bridges HR, Jones AJ, Pollak MN, Hirst J . Effects of metformin and other biguanides on oxidative phosphorylation in mitochondria. Biochem J 2014; 462: 475–487.
Pollak MN . Investigating metformin for cancer prevention and treatment: the end of the beginning. Cancer Discov 2012; 2: 778–790.
Madiraju AK, Erion DM, Rahimi Y, Zhang XM, Braddock DT, Albright RA et al. Metformin suppresses gluconeogenesis by inhibiting mitochondrial glycerophosphate dehydrogenase. Nature 2014; 510: 542–546.
Ma J, Li H, Giovannucci E, Mucci L, Qiu W, Nguyen PL et al. Prediagnostic body-mass index, plasma C-peptide concentration, and prostate cancer-specific mortality in men with prostate cancer: a long-term survival analysis. Lancet Oncol 2008; 9: 1039–1047.
Pollak M . Overcoming drug development bottlenecks with repurposing: repurposing biguanides to target energy metabolism for cancer treatment. Nat Med 2014; 20: 591–593.
Wang DS, Jonker JW, Kato Y, Kusuhara H, Schinkel AH, Sugiyama Y . Involvement of organic cation transporter 1 in hepatic and intestinal distribution of metformin. J Pharmacol Exp Therap 2002; 302: 510–515.
Kimura N, Okuda M, Inui K . Metformin transport by renal basolateral organic cation transporter hOCT2. Pharm Res 2005; 22: 255–259.
Muller J, Lips KS, Metzner L, Neubert RH, Koepsell H, Brandsch M . Drug specificity and intestinal membrane localization of human organic cation transporters (OCT). Biochem Pharmacol 2005; 70: 1851–1860.
Koepsell H . Polyspecific organic cation transporters: their functions and interactions with drugs. Trends Pharmacol Sci 2004; 25: 375–381.
Koepsell H, Lips K, Volk C . Polyspecific organic cation transporters: structure, function, physiological roles, and biopharmaceutical implications. Pharm Res 2007; 24: 1227–1251.
Meyer ZU, Schwabedissen HE, Verstuyft C, Kroemer HK, Becquemont L, Kim RB . Human multidrug and toxin extrusion 1 (MATE1/SLC47A1) transporter: functional characterization, interaction with OCT2 (SLC22A2), and single nucleotide polymorphisms. Am J Physiol Renal Physiol 2010; 298: F997–F1005.
Shu Y, Brown C, Castro RA, Shi RJ, Lin ET, Owen RP et al. Effect of genetic variation in the organic cation transporter 1, OCT1, on metformin pharmacokinetics. Clin Pharmacol Ther 2008; 83: 273–280.
Tzvetkov MV, Vormfelde SV, Balen D, Meineke I, Schmidt T, Sehrt D et al. The effects of genetic polymorphisms in the organic cation transporters OCT1, OCT2, and OCT3 on the renal clearance of metformin. Clin Pharmacol Ther 2009; 86: 299–306.
Christensen MM, Brasch-Andersen C, Green H, Nielsen F, Damkier P, Beck-Nielsen H et al. The pharmacogenetics of metformin and its impact on plasma metformin steady-state levels and glycosylated hemoglobin A1c. Pharmacogenet Genom 2011; 21: 837–850.
Christensen MM, Pedersen RS, Stage TB, Brasch-Andersen C, Nielsen F, Damkier P et al. A gene–gene interaction between polymorphisms in the OCT2 and MATE1 genes influences the renal clearance of metformin. Pharmacogenet Genom 2013; 23: 526–534.
Stocker SL, Morrissey KM, Yee SW, Castro RA, Xu L, Dahlin A et al. The effect of novel promoter variants in MATE1 and MATE2 on the pharmacokinetics and pharmacodynamics of metformin. Clin Pharmacol Therap 2013; 93: 186–194.
Shu Y, Sheardown SA, Brown C, Owen RP, Zhang S, Castro RA et al. Effect of genetic variation in the organic cation transporter 1 (OCT1) on metformin action. J Clin Invest 2007; 117: 1422–1431.
Becker ML, Visser LE, van Schaik RH, Hofman A, Uitterlinden AG, Stricker BH . Genetic variation in the multidrug and toxin extrusion 1 transporter protein influences the glucose-lowering effect of metformin in patients with diabetes: a preliminary study. Diabetes 2009; 58: 745–749.
Becker ML, Visser LE, van Schaik RH, Hofman A, Uitterlinden AG, Stricker BH . Genetic variation in the organic cation transporter 1 is associated with metformin response in patients with diabetes mellitus. Pharmacogenom J 2009; 9: 242–247.
Becker ML, Visser LE, van Schaik RH, Hofman A, Uitterlinden AG, Stricker BH . Interaction between polymorphisms in the OCT1 and MATE1 transporter and metformin response. Pharmacogenet Genom 2010; 20: 38–44.
Tarasova L, Kalnina I, Geldnere K, Bumbure A, Ritenberga R, Nikitina-Zake L et al. Association of genetic variation in the organic cation transporters OCT1, OCT2 and multidrug and toxin extrusion 1 transporter protein genes with the gastrointestinal side effects and lower BMI in metformin-treated type 2 diabetes patients. Pharmacogenet Genom 2012; 22: 659–666.
Tkac I, Klimcakova L, Javorsky M, Fabianova M, Schroner Z, Hermanova H et al. Pharmacogenomic association between a variant in SLC47A1 gene and therapeutic response to metformin in type 2 diabetes. Diabetes Obes Metab 2013; 15: 189–191.
Toyama K, Yonezawa A, Masuda S, Osawa R, Hosokawa M, Fujimoto S et al. Loss of multidrug and toxin extrusion 1 (MATE1) is associated with metformin-induced lactic acidosis. Br J Pharmacol 2012; 166: 1183–1191.
Rizos CV, Elisaf MS . Metformin and cancer. Eur J Pharmacol 2013; 705: 96–108.
International Conference on Harmonisation of technical requirements for registration of pharmaceuticals for human u. ICH harmonized tripartite guideline: Guideline for Good Clinical Practice. J Postgrad Med 2001; 47: 45–50.
Rothermundt C, Hayoz S, Templeton AJ, Winterhalder R, Strebel RT, Bartschi D et al. Metformin in chemotherapy-naive castration-resistant prostate cancer: A Multicenter Phase 2 Trial (SAKK 08/09). Eur Urol 2014; 66: 468–474.
Scher HI, Halabi S, Tannock I, Morris M, Sternberg CN, Carducci MA et al. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol 2008; 26: 1148–1159.
Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC . Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985; 28: 412–419.
Algire C, Amrein L, Bazile M, David S, Zakikhani M, Pollak M . Diet and tumor LKB1 expression interact to determine sensitivity to anti-neoplastic effects of metformin in vivo. Oncogene 2011; 30: 1174–1182.
Birsoy K, Possemato R, Lorbeer FK, Bayraktar EC, Thiru P, Yucel B et al. Metabolic determinants of cancer cell sensitivity to glucose limitation and biguanides. Nature 2014; 508: 108–112.
Shackelford DB, Abt E, Gerken L, Vasquez DS, Seki A, Leblanc M et al. LKB1 inactivation dictates therapeutic response of non-small cell lung cancer to the metabolism drug phenformin. Cancer Cell 2013; 23: 143–158.
DeCensi A, Puntoni M, Gandini S, Guerrieri-Gonzaga A, Johansson HA, Cazzaniga M et al. Differential effects of metformin on breast cancer proliferation according to markers of insulin resistance and tumor subtype in a randomized presurgical trial. Breast Cancer Res Treat 2014; 148: 81–90.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Joerger, M., van Schaik, R., Becker, M. et al. Multidrug and toxin extrusion 1 and human organic cation transporter 1 polymorphisms in patients with castration-resistant prostate cancer receiving metformin (SAKK 08/09). Prostate Cancer Prostatic Dis 18, 167–172 (2015). https://doi.org/10.1038/pcan.2015.8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/pcan.2015.8