Abstract
Background:
Ketoconazole is a well-known CYP17-targeted systemic treatment for castration-resistant prostate cancer (CRPC). However, most of the published data has been in the pre-chemotherapy setting; its efficacy in the post-chemotherapy setting has not been as widely described. Chemotherapy-naïve patients treated with attenuated doses of ketoconazole (200–300 mg three times daily) had PSA response rate (>50% decline) of 21–62%. We hypothesized that low-dose ketoconazole would likewise possess efficacy and tolerability in the CRPC post-chemotherapy state.
Methods:
Men with CRPC and performance status 0–3, adequate organ function and who had received prior docetaxel were treated with low-dose ketoconazole (200 mg orally three times daily) and hydrocortisone (20 mg PO qAM and 10 mg PO qPM) until disease progression. Primary endpoint was PSA response rate (>50% reduction from baseline) where a rate of 25% was to be considered promising for further study (versus a null rate of <5%); 25 patients were required. Secondary endpoints included PSA response >30% from baseline, progression-free survival (PFS), duration of stable disease and evaluation of adverse events (AEs).
Results:
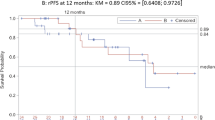
Thirty patients were accrued with median age of 72 years (range 55–86) and median pre-treatment PSA of 73 ng ml−1 (range 7–11,420). Twenty-nine patients were evaluable for response and toxicity. PSA response (>50% reduction) was seen in 48% of patients; PSA response (>30% reduction) was seen in 59%. Median PFS was 138 days; median duration of stable disease was 123 days. Twelve patients experienced grade 3 or 4 AEs. Of the 17 grade 3 AEs, only 3 were attributed to treatment. None of the two grade 4 AEs were considered related to treatment.
Conclusions:
In docetaxel pre-treated CRPC patients, low-dose ketoconazole and hydrocortisone is a well-tolerated, relatively inexpensive and clinically active treatment option. PSA response to low-dose ketoconazole appears historically comparable to that of abiraterone in this patient context. A prospective, randomized study of available post-chemotherapy options is warranted to assess comparative efficacy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 4 print issues and online access
$259.00 per year
only $64.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Chen RJ, Lee WS, Liang YC, Lin JK, Wang YJ, Lin CH et al. Ketoconazole induces G0/G1 arrest in human colorectal and hepatocellular carcinoma cell lines. Toxicol Appl Pharmacol 2000; 169: 132–141.
Eichenberger T, Trachtenberg J, Toor P, Keating A . Ketoconazole: a possible direct cytotoxic effect on prostate carcinoma cells. J Urol 1989; 141: 190–191.
Rochlitz CF, Damon LE, Russi MB, Geddes A, Cadman EC . Cytotoxicity of ketoconazole in malignant cell lines. Cancer Chemother Pharmacol 1988; 21: 319–322.
Keizman D, Huang P, Carducci MA, Eisenberger MA . Contemporary experience with ketoconazole in patients with metastatic castration-resistant prostate cancer: clinical factors associated with PSA response and disease progression. Prostate 2012; 72: 461–467.
Harris KA, Weinberg V, Bok RA, Kakefuda M, Small EJ . Low dose ketoconazole with replacement doses of hydrocortisone in patients with progressive androgen independent prostate cancer. J Urol 2002; 168: 542–545.
Nakabayashi M, Xie W, Regan MM, Jackman DM, Kantoff PW, Oh WK et al. Response to low-dose ketoconazole and subsequent dose escalation to high-dose ketoconazole in patients with androgen-independent prostate cancer. Cancer 2006; 107: 975–981.
Ngo LS, Yeo A, Wong AS, Tay MH . Efficacy of low-dose ketoconazole in hormone refractory prostate cancer patients at the National Cancer Centre and The Cancer Institute, Singapore. Ann Acad Med Singapore 2007; 36: 811–814.
Petrylak DP, Tangen CM, Hussain MH, Lara PN Jr, Jones JA, Taplin ME et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med 2004; 351: 1513–1520.
Tannock IF, de Wit R, Berry WR, Horti J, Pluzanska A, Chi KN et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med 2004; 351: 1502–1512.
de Bono JS, Logothetis CJ, Molina A, Fizazi K, North S, Chu L et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med 2011; 364: 1995–2005.
Ryan CJ, Smith MR, de Bono JS, Molina A, Logothetis CJ, de Souza P et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med 2013; 368: 138–148.
Scher HI, Fizazi K, Saad F, Taplin ME, Sternberg CN, Miller K et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med 2012; 367: 1187–1197.
Galsky MD, Simon K, Sonpavde G, Hutson TE, Fleming M, Kondagunta GV et al. Ketoconazole retains activity in patients with docetaxel-refractory prostate cancer. Ann Oncol 2009; 20: 965–966.
Nakabayashi M, Oh WK, Jacobus S, Regan MM, Taplin ME, Kantoff PW et al. Activity of ketoconazole after taxane-based chemotherapy in castration-resistant prostate cancer. BJU Int 2010; 105: 1392–1396.
Small EJ, Halabi S, Dawson NA, Stadler WM, Rini BI, Picus J et al. Antiandrogen withdrawal alone or in combination with ketoconazole in androgen-independent prostate cancer patients: a phase III trial (CALGB 9583). J Clin Oncol 2004; 22: 1025–1033.
Peer A, Gottfried M, Sinibaldi V, Carducci MA, Eisenberger MA, Sella A et al. Comparison of abiraterone acetate versus ketoconazole in patients with metastatic castration resistant prostate cancer refractory to docetaxel. Prostate 2014; 74: 433–440.
Armstrong AJ, Garrett-Mayer E, Ou Yang YC, Carducci MA, Tannock I, de Wit R et al. Prostate-specific antigen and pain surrogacy analysis in metastatic hormone-refractory prostate cancer. J Clin Oncol 2007; 25: 3965–3970.
Hussain M, Goldman B, Tangen C, Higano CS, Petrylak DP, Wilding G et al. Prostate-specific antigen progression predicts overall survival in patients with metastatic prostate cancer: data from Southwest Oncology Group Trials 9346 (Intergroup Study 0162) and 9916. J Clin Oncol 2009; 27: 2450–2456.
Kapoor A . What's new in prostate cancer research? Highlights of GU-ASCO 2014. Can Urol Assoc J 2014; 8: S8–S12.
Mezynski J, Pezaro C, Bianchini D, Zivi A, Sandhu S, Thompson E et al. Antitumour activity of docetaxel following treatment with the CYP17A1 inhibitor abiraterone: clinical evidence for cross-resistance? Ann Oncol 2012; 23: 2943–2947.
Loriot Y, Bianchini D, Ileana E, Sandhu S, Patrikidou A, Pezaro C, Albiges L et al. Antitumour activity of abiraterone acetate against metastatic castration-resistant prostate cancer progressing after docetaxel and enzalutamide (MDV3100). Ann Oncol 2013; 24: 1807–1812.
Aggarwal R, Halabi S, Kelly WK, George D, Mahoney JF, Millard F et al. The effect of prior androgen synthesis inhibition on outcomes of subsequent therapy with docetaxel in patients with metastatic castrate-resistant prostate cancer: results from a retrospective analysis of a randomized phase 3 clinical trial (CALGB 90401) (Alliance). Cancer 2013; 119: 3636–3643.
Zhong L, Pon V, Srinivas S, Nguyen N, Frear M, Kwon S et al. Therapeutic options in docetaxel-refractory metastatic castration-resistant prostate cancer: a cost-effectiveness analysis. PLoS One 2013; 8: e64275.
Small EJ, Meyer M, Marshall ME, Reyno LM, Meyers FJ, Natale RB et al. Suramin therapy for patients with symptomatic hormone-refractory prostate cancer: results of a randomized phase III trial comparing suramin plus hydrocortisone to placebo plus hydrocortisone. J Clin Oncol 2000; 18: 1440–1450.
Acknowledgements
This study was supported by the VA Career Development Award-2 (PI: Pan), VA Merit (PI: Pan; Grant #1I01BX001784) and the NCI Cancer Center Support Grant (PI: de Vere White). Statistical Support was provided by the Biostatistics Shared Resource through the UC Davis Comprehensive Cancer Center Support Grant, P30CA093373-06. Clinical Trials Registration: ClinicalTrials.gov Identifiers NCT00895310.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Annual Meeting of the American Society of Clinical Oncology Genitourinary Symposium, January 2014, San Francisco, CA, USA.
Rights and permissions
About this article
Cite this article
Lo, E., Beckett, L., Pan, CX. et al. Prospective evaluation of low-dose ketoconazole plus hydrocortisone in docetaxel pre-treated castration-resistant prostate cancer patients. Prostate Cancer Prostatic Dis 18, 144–148 (2015). https://doi.org/10.1038/pcan.2015.2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/pcan.2015.2
This article is cited by
-
The role of ketoconazole in current prostate cancer care
Nature Reviews Urology (2018)