Abstract
Background:
To evaluate the safety and clinical feasibility of focal irreversible electroporation (IRE) of the prostate.
Methods:
We assessed the toxicity profile and functional outcomes of consecutive patients undergoing focal IRE for localised prostate cancer in two centres. Eligibility was assessed by multi-parametric magnetic resonance imaging (mpMRI) and targeted and/or template biopsy. IRE was delivered under transrectal ultrasound guidance with two to six electrodes positioned transperineally within the cancer lesion. Complications were recorded and scored accordingly to the NCI Common Terminology Criteria for Adverse Events; the functional outcome was physician reported in all patients with at least 6 months follow-up. A contrast-enhanced MRI 1 week after the procedure was carried out to assess treatment effect with a further mpMRI at 6 months to rule out evidence of residual visible cancer.
Results:
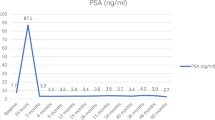
Overall, 34 patients with a mean age of 65 years (s.d.=±6) and a median PSA of 6.1 ng ml−1 (interquartile range (IQR)= 4.3–7.7) were included. Nine (26%), 24 (71%) and 1 (3%) men had low, intermediate and high risk disease, respectively (D’Amico criteria). After a median follow-up of 6 months (range 1–24), 12 grade 1 and 10 grade 2 complications occurred. No patient had grade >/= 3 complication. From a functional point of view, 100% (24/24) patients were continent and potency was preserved in 95% (19/20) men potent before treatment. The volume of ablation was a median 12 ml (IQR=5.6–14.5 ml) with the median PSA after 6 months of 3.4 ng ml−1 (IQR=1.9–4.8 ng ml−1). MpMRI showed suspicious residual disease in six patients, of whom four (17%) underwent another form of local treatment.
Conclusions:
Focal IRE has a low toxicity profile with encouraging genito-urinary functional outcomes. Further prospective development studies are needed to confirm the functional outcomes and to explore the oncological potential.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 4 print issues and online access
$259.00 per year
only $64.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Wilt TJ, Brawer MK, Jones KM, Barry MJ, Aronson WJ, Fox S et al. Radical prostatectomy versus observation for localized prostate cancer. N Engl J Med 2012; 367: 203–213.
Ahmed HU, Freeman A, Kirkham A, Sahu M, Scott R, Allen C et al. Focal therapy for localized prostate cancer: a phase I/II trial. J Urol 2011; 185: 1246–1254.
Ahmed HU, Hindley RG, Dickinson L, Freeman A, Kirkham AP, Sahu M et al. Focal therapy for localised unifocal and multifocal prostate cancer: a prospective development study. Lancet Oncol 2012; 13: 622–632.
Emberton M . A European randomised phase 3 study to assess the efficacy and safety of TOOKAD® soluble for localised prostate cancer compared to active surveillance. ClinicalTrials.gov Identifier: NCT01310894, 31 October 2012.
Bahn D, de Castro Abreu AL, Gill IS, Hung AJ, Silverman P, Gross ME et al. Focal cryotherapy for clinically unilateral, low-intermediate risk prostate cancer in 73 men with a median follow-up of 3.7 years. Eur Urol 2012; 62: 55–63.
Oto A, Sethi I, Karczmar G, McNichols R, Ivancevic MK, Stadler WM et al. MR imaging-guided focal laser ablation for prostate cancer: phase I trial. Radiology 2013; 267: 932–940.
Barret E, Ahallal Y, Sanchez-Salas R, Galiano M, Cosset JM, Validire P et al. Morbidity of focal therapy in the treatment of localized prostate cancer. Eur Urol 2013; 63: 618–622.
Lindner U, Weersink RA, Haider MA, Gertner MR, Davidson SR, Atri M et al. Image guided photothermal focal therapy for localized prostate cancer: phase I trial. J Urol 2009; 182: 1371–1377.
Valerio M, Ahmed HU, Emberton M, Lawrentschuk N, Lazzeri M, Montironi R et al. The role of focal therapy in the management of localised prostate cancer: a systematic review. Eur Urol 2013; S0302-2838: 00557–5.
Pech M, Janitzky A, Wendler JJ, Strang C, Blaschke S, Dudeck O et al. Irreversible electroporation of renal cell carcinoma: a first-in-man phase I clinical study. Cardiovasc Intervent Radiol 2011; 34: 132–138.
Martin RC 2nd, McFarland K, Ellis S, Velanovich V . Irreversible electroporation therapy in the management of locally advanced pancreatic adenocarcinoma. J A Coll Surg 2012; 215: 361–369.
Charpentier KP . Irreversible electroporation for the ablation of liver tumors: are we there yet? Arch Surg 2012; 147: 1053–1061.
Philips P, Hays D, Martin RC . Irreversible electroporation ablation (IRE) of unresectable soft tissue tumors: learning curve evaluation in the first 150 patients treated. PloS One 2013; 8: e76260.
Onik G, Rubinsky B . Irreversible Electroporation: First Patient Experience Focal Therapy of Prostate Cancer. Series in Biomedical Engineering. Berlin Heidelberg: Springer, 2010, pp 235–247.
Neal RE 2nd, Millar JL, Kavnoudias H, Royce P, Rosenfeldt F, Pham A et al. In vivo characterization and numerical simulation of prostate properties for non-thermal irreversible electroporation ablation. Prostate 2014; 74: 458–468.
Rubinsky J, Onik G, Mikus P, Rubinsky B . Optimal parameters for the destruction of prostate cancer using irreversible electroporation. J Urol 2008; 180: 2668–2674.
Faroja M, Ahmed M, Appelbaum L, Ben-David E, Moussa M, Sosna J et al. Irreversible electroporation ablation: is all the damage nonthermal? Radiology 2013; 266: 462–470.
Emberton M . A prospective development study evaluating focal therapy using irreversible electroporation (Nanoknife®) in men with localised prostate cancer. ClinicalTrials.gov Identifier: NCT01726894, 31 October 2012.
Punwani S, Emberton M, Walkden M, Sohaib A, Freeman A, Ahmed H et al. Prostatic cancer surveillance following whole-gland high-intensity focused ultrasound: comparison of MRI and prostate-specific antigen for detection of residual or recurrent disease. Bri J Radiol 2012; 85: 720–728.
Gupta RT, Kauffman CR, Polascik TJ, Taneja SS, Rosenkrantz AB . The state of prostate MRI in 2013. Oncology (Williston Park) 2013; 27: 262–270.
Turkbey B, Choyke PL . Multiparametric MRI and prostate cancer diagnosis and risk stratification. Curr Opin Urol 2012; 22: 310–315.
Arumainayagam N, Ahmed HU, Moore CM, Freeman A, Allen C, Sohaib SA et al. Multiparametric MR imaging for detection of clinically significant prostate cancer: a validation cohort study with transperineal template prostate mapping as the reference standard. Radiology 2013; 268: 761–769.
Thompson JE, Moses D, Shnier R, Brenner P, Delprado W, Ponsky L et al. Multiparametric magnetic resonance imaging guided diagnostic biopsy detects significant prostate cancer and could reduce unnecessary biopsies and over detection: a prospective study. J Urol (e-pub ahead of print 8 February 2014; doi: 10.1016/j.juro.2014.01.014.
Onik G, Mikus P, Rubinsky B . Irreversible electroporation: implications for prostate ablation. Technol Cancer Res Treat 2007; 6: 295–300.
Li W, Fan Q, Ji Z, Qiu X, Li Z . The effects of irreversible electroporation (IRE) on nerves. PloS One 2011; 6: e18831.
Tsivian M, Polascik TJ . Bilateral focal ablation of prostate tissue using low-energy direct current (LEDC): a preclinical canine study. BJU Int 2013; 112: 526–530.
McCulloch P, Cook JA, Altman DG, Heneghan C, Diener MK . IDEAL framework for surgical innovation 1: the idea and development stages. BMJ 2013; 346: f3012.
Ergina PL, Barkun JS, McCulloch P, Cook JA, Altman DG . IDEAL framework for surgical innovation 2: observational studies in the exploration and assessment stages. BMJ 2013; 346: f3011.
Cook JA, McCulloch P, Blazeby JM, Beard DJ, Marinac-Dabic D, Sedrakyan A . IDEAL framework for surgical innovation 3: randomised controlled trials in the assessment stage and evaluations in the long term study stage. BMJ 2013; 346: f2820.
Acknowledgements
The SICPA foundation supports the ongoing fellowship and PhD programme of M. Valerio. M. Emberton and H.U. Ahmed acknowledge funding from the Medical Research Council (UK), the Pelican Cancer Foundation charity, Prostate Cancer UK, St Peters Trust charity, Prostate Cancer Research Centre, the Wellcome Trust, National Institute of Health Research-Health Technology Assessment programme, and the US National Institute of Health-National Cancer Institute. M. Emberton receives funding in part from the UK National Institute of Health Research UCLH/UCL Comprehensive Biomedical Research Centre.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
M. Valerio has received funding for conference attendance from Geoscan Medical. M. Emberton and H.U. Ahmed receive funding from USHIFU, GSK, AngioDynamics and Advanced Medical Diagnostics for clinical trials. M. Emberton is a paid consultant to AngioDynamics, Steba Biotech and SonaCare Medical (previously called USHIFU). Both have previously received consultancy payments from Oncura/GE Healthcare and Steba Biotech. L. Dickinson has received trial funding support from SonaCare Medical and consultancy fees from SonaCare Medical and Oncura. None of these sources had any input whatsoever into this article. The remaining authors declare no conflicts of interest.
Rights and permissions
About this article
Cite this article
Valerio, M., Stricker, P., Ahmed, H. et al. Initial assessment of safety and clinical feasibility of irreversible electroporation in the focal treatment of prostate cancer. Prostate Cancer Prostatic Dis 17, 343–347 (2014). https://doi.org/10.1038/pcan.2014.33
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/pcan.2014.33
This article is cited by
-
Cryoablation, high-intensity focused ultrasound, irreversible electroporation, and vascular-targeted photodynamic therapy for prostate cancer: a systemic review and meta-analysis
International Journal of Clinical Oncology (2021)
-
MRI guided procedure planning and 3D simulation for partial gland cryoablation of the prostate: a pilot study
3D Printing in Medicine (2020)
-
Ablation energies for focal treatment of prostate cancer
World Journal of Urology (2019)
-
Irreversible Electroporation for the Ablation of Prostate Cancer
Current Urology Reports (2019)
-
Irreversible electroporation for catheter-based cardiac ablation: a systematic review of the preclinical experience
Journal of Interventional Cardiac Electrophysiology (2019)