Abstract
Background:
About 20% of patients with prostate cancer have an ECOG performance status (PS) ⩾2 at diagnosis. We investigate if current treatment options for castration-resistant prostate cancer (CRPC) may decrease the risk of death even in patients with ECOG PS of 2.
Methods:
PubMed was reviewed for phase III randomized trials in patients with CRPC progressed after docetaxel chemotherapy. Characteristics of each study and the relative hazard ratio (HR) for overall survival and 95% confidence interval (CI) were collected. Summary HR was calculated using random- or fixed-effects models depending on the heterogeneity of included studies.
Results:
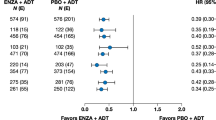
A total of 3,149 patients was available for meta-analysis. In the overall population, the experimental treatments decrease the risk of death by 31% (HR=0.69; 95% CI: 0.63–0.76; P<0.001). The activity of experimental treatments was similar in 2,859 patients with ECOG-PS=0 or 1 with a reduced risk of death of 31% (HR=0.69; 95% CI: 0.62–0.76). A total of 290 patients (9.2%) had ECOG-PS=2 and experimental treatments decreased the risk of death by 26% (HR=0.74; 95% CI: 0.56–0.98; P=0.035) compared with the controls even in this sub-group. When patients were stratified by type of treatment, the reduction of the risk of death was confirmed for hormonal therapies: abiraterone and enzalutamide (HR=0.72; 95% CI: 0.52–0.99; P=0.046), but not for chemotherapy (HR=0.81; 95% CI: 0.48–1.37; P=0.43).
Conclusions:
We believe this is the first study reporting a benefit in second-line setting for CRPC patients previously treated with docetaxel and poor PS.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 4 print issues and online access
$259.00 per year
only $64.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
American Cancer Society. Cancer Facts & Figures 2012 American Cancer Society: Atlanta, Georgia, USA.
Tannock IF, de Wit R, Berry WR, Horti J, Pluzanska A, Chi KN et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med 2004; 351: 1502–1512.
Petrylak DP, Tangen CM, Hussain MH, Lara PN Jr, Jones JA, Taplin ME et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med 2004; 351: 1513–1520.
Altavilla A, Iacovelli R, Procopio G, Alesini D, Risi E, Campennì GM et al. Medical strategies for treatment of castration resistant prostate cancer (CRPC) docetaxel resistant. Cancer Biol Ther 2012; 13: 1001–1008.
Paoli CJ, Bach BA, Tsai KT, Wong B . A retrospective study of performance status in oncology patients at diagnosis and over the first year in routine clinical practice. J Clin Oncol 2011; 29, (suppl; abstr e16521).
Oosterlinck W, Mattelaer J, Derde MP, Kaufman L . Prognostic factors in advanced prostatic carcinoma treated with total androgen blockade. Flutamide with orchiectomy or with LHRH analogues. A Belgian multicentric study of 546 patients. Acta Urol Belg 1995; 63: 1–9.
Halabi S, Vogelzang NJ, Kornblith AB, Ou SS, Kantoff PW, Dawson NA, Small EJ . Pain predicts overall survival in men with metastatic castration-refractory prostate cancer. J Clin Oncol 2008; 26: 2544–2549.
Omlin A, Pezaro C, Mukherji D, Mulick Cassidy A, Sandhu S, Bianchini D et al. Improved survival in a cohort of trial participants with metastatic castration-resistant prostate cancer demonstrates the need for updated prognostic nomograms. Eur Urol 2013; 64: 300–306.
Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, Carbone PP . Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 1982; 5: 649–655.
Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, McQuay HJ . Assessing the quality of reports of randomized clinical trials: Is blinding necessary? Control Clin Trials 1996; 17: 1–12.
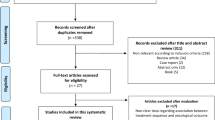
Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009; 339: b2535.
Morris JA, Gardner MJ . Calculating confidence intervals for relative risks (odds ratios) and standardised ratios and rates. Br Med J 1988; 296: 1313–1316.
Higgins JP, Thompson SG, Deeks JJ, Altman DG . Measuring inconsistency in meta-analyses. BMJ 2003; 327: 557–560.
Der Simonian R, Laird N . Meta-analysis in clinical trials. Control Clin Trials 1986; 7: 177–188.
Begg CB, Mazumdar M . Operating characteristics of a rank correlation test for publication bias. Biometrics 1994; 50: 1088–1101.
Egger M, Davey Smith G, Schneider M, Minder C . Bias in meta-analysis detected by a simple, graphical test. BMJ 1997; 315: 629–634.
Review Manager (RevMan) [Computer program]. Version 5.2. The Nordic Cochrane Centre. The Cochrane Collaboration. Copenhagen, 2012.
Sternberg CN, Petrylak DP, Sartor O, Witjes JA, Demkow T, Ferrero JM et al. Multinational, double-blind, phase III study of prednisone and either satraplatin or placebo in patients with castrate-refractory prostate cancer progressing after prior chemotherapy: the SPARC trial. J Clin Oncol 2009; 27: 5431–5438.
de Bono JS, Oudard S, Ozguroglu M, Hansen S, Machiels JP, Kocak I et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet 2010; 376: 1147–1154.
Fizazi K, Scher HI, Molina A, Logothetis CJ, Chi KN, Jones RJ et al. Abiraterone acetate for treatment of metastatic castration-resistant prostate cancer: final overall survival analysis of the COU-AA-301 randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol 2012; 13: 983–992.
Scher HI, Fizazi K, Saad F, Taplin ME, Sternberg CN, Miller K et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med 2012; 367: 1187–1197.
Morabito A, Gebbia V, Di Maio M, Cinieri S, Viganò MG, Bianco R et al. Randomized phase III trial of gemcitabine and cisplatin vs gemcitabine alone in patients with advanced non-small cell lung cancer and a performance status of 2: The CAPPA-2 study. Lung Cancer 2013; 81: 77–83.
Zukin M, Barrios CH, Rodrigues Pereira J, De Albuquerque Ribeiro R, de Mendonça Beato CA, do Nascimento YN et al. Randomized phase III trial of single-agent pemetrexed versus carboplatin and pemetrexed in patients with advanced non-small cell lung cancer and Eastern Cooperative Oncology Group performance status of 2. J Clin Oncol 2013; (Epub ahead of print).
Jouveshomme S, Canoui-Poitrine F, Le Thuaut A, Bastuji-Garin S . Results of platinum-based chemotherapy in unselected performance status (PS) 2 patients with advanced non-small cell lung cancer: a cohort study. Med Oncol 2013; 30: 544–553.
National Comprehensive Cancer Network. Prostate Cancer. http://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf. . Accessed on 20 January 2012.
Ryan CJ, Smith MR, de Bono JS, Molina A, Logothetis CJ, de Souza P et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med 2013; 368: 138–148.
de Bono JS, Logothetis CJ, Molina A, Fizazi K, North S, Chu L et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med 2011; 364: 1995–2005.
Logothetis CJ, Basch E, Molina A, Fizazi K, North SA, Chi KN et al. Effect of abiraterone acetate and prednisone compared with placebo and prednisone on pain control and skeletal-related events in patients with metastatic castration-resistant prostate cancer: exploratory analysis of data from the COU-AA-301 randomised trial. Lancet Oncol 2012; 13: 1210–1217.
Sternberg CN, Molina A, North S, Mainwaring P, Fizazi K, Hao Y et al. Effect of abiraterone acetate on fatigue in patients with metastatic castration-resistant prostate cancer after docetaxel chemotherapy. Ann Oncol 2012; 24 (4): 1017–1025.
Kantoff PW, Halabi S, Conaway M, Picus J, Kirshner J, Hars V et al. Hydrocortisone with or without mitoxantrone in men with hormone-refractory prostate cancer: results of the cancer and leukemia group B 9182 study. J Clin Oncol 1999; 17: 2506–2513.
Tannock IF, Osoba D, Stockler MR, Ernst DS, Neville AJ, Moore MJ et al. Chemotherapy with mitoxantrone plus prednisone or prednisone alone for symptomatic hormoneresistant prostate cancer: a Canadian randomized trial with palliative end points. J Clin Oncol 1996; 14: 1756–1764.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Iacovelli, R., Altavilla, A., Procopio, G. et al. Are post-docetaxel treatments effective in patients with castration-resistant prostate cancer and performance of 2? A meta-analysis of published trials. Prostate Cancer Prostatic Dis 16, 323–327 (2013). https://doi.org/10.1038/pcan.2013.20
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/pcan.2013.20
Keywords
This article is cited by
-
Longitudinal model–based meta-analysis for survival probabilities in patients with castration-resistant prostate cancer
European Journal of Clinical Pharmacology (2020)
-
Chemotherapy management for unfit patients with metastatic castration-resistant prostate cancer
Clinical and Translational Oncology (2019)