Abstract
Background:
Intermediate end points are desirable to expedite the integration of neoadjuvant systemic therapy into the treatment strategy for high-risk localized prostate cancer. Endorectal magnetic resonance imaging at 1.5 Tesla (1.5T erMRI) response has been utilized as an end point in neoadjuvant trials but has not been correlated with clinical outcomes.
Methods:
Data were pooled from two trials exploring neoadjuvant chemotherapy in high-risk localized prostate cancer. Trial 1 explored docetaxel for 6 months and Trial 2 explored docetaxel plus bevacizumab for 4.5 months, both before radical prostatectomy. erMRI was done at baseline and end of chemotherapy. 1.5T erMRI response, based upon T2W sequences, was recorded. Multivariable Cox regression was undertaken to evaluate the association between clinical parameters and biochemical recurrence.
Results:
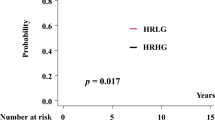
There were 53 evaluable patients in the combined analysis: 20 (33%) achieved a PSA response, 16 (27%) achieved an erMRI partial response and 24 (40%) achieved an erMRI minor response. Median follow-up was 4.2 years, and 33 of 53 evaluable (62%) patients developed biochemical recurrence. On multivariable analysis, PSA response did not correlate with biochemical recurrence (hazard ratio=0.58, 95% confidence interval (CI) 0.25–1.33) and paradoxically erMRI response was associated with a significantly shorter time to biochemical recurrence (hazard ratio=2.47, 95% CI 1.00–6.13).
Conclusions:
Response by 1.5T erMRI does not correlate with a decreased likelihood of biochemical recurrence in patients with high-risk localized prostate cancer treated with neoadjuvant docetaxel and may be associated with inferior outcomes. These data do not support the use of 1.5T erMRI response as a primary end point in neoadjuvant chemotherapy trials.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 4 print issues and online access
$259.00 per year
only $64.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Siegel R, Naishadham D, Jemal A . Cancer statistics, 2012. CA Cancer J Clin 2012; 62: 10–29.
Ganz PA, Barry JM, Burke W, Col NF, Corso PS, Dodson E et al. National Institutes of Health State-of-the-Science Conference: role of active surveillance in the management of men with localized prostate cancer. Ann Int Med 2012; 156: 591–595.
Kibel AS, Ciezki JP, Klein EA, Reddy CA, Lubahn JD, Haslag-Minoff J et al. Survival among men with clinically localized prostate cancer treated with radical prostatectomy or radiation therapy in the prostate specific antigen era. J Urol 2012; 187: 1259–1265.
D'Amico AV, Whittington R, Malkowicz SB, Schultz D, Blank K, Broderick GA et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. Jama 1998; 280: 969–974.
Kattan MW, Eastham JA, Stapleton AM, Wheeler TM, Scardino PT . A preoperative nomogram for disease recurrence following radical prostatectomy for prostate cancer. J Natl Cancer Inst 1998; 90: 766–771.
Partin AW, Kattan MW, Subong EN, Walsh PC, Wojno KJ, Oesterling JE et al. Combination of prostate-specific antigen, clinical stage, and Gleason score to predict pathological stage of localized prostate cancer. A multi-institutional update. Jama 1997; 277: 1445–1451.
Stephenson AJ, Kattan MW, Eastham JA, Bianco FJ Jr, Yossepowitch O, Vickers AJ et al. Prostate cancer-specific mortality after radical prostatectomy for patients treated in the prostate-specific antigen era. J Clin Oncol 2009; 27: 4300–4305.
Chi KN, Chin JL, Winquist E, Klotz L, Saad F, Gleave ME . Multicenter phase II study of combined neoadjuvant docetaxel and hormone therapy before radical prostatectomy for patients with high risk localized prostate cancer. J Urol 2008; 180: 565–570 discussion 70.
Dreicer R, Magi-Galluzzi C, Zhou M, Rothaermel J, Reuther A, Ulchaker J et al. Phase II trial of neoadjuvant docetaxel before radical prostatectomy for locally advanced prostate cancer. Urology 2004; 63: 1138–1142.
Febbo PG, Richie JP, George DJ, Loda M, Manola J, Shankar S et al. Neoadjuvant docetaxel before radical prostatectomy in patients with high-risk localized prostate cancer. Clin Cancer Res 2005; 11: 5233–5240.
Prentice RL . Surrogate endpoints in clinical trials: definition and operational criteria. Stat Med 1989; 8: 431–440.
Esserman LJ, Berry DA, Demichele A, Carey L, Davis SE, Buxton M et al. Pathologic complete response predicts recurrence-free survival more effectively by cancer subset: results from the I-SPY 1 TRIAL--CALGB 150007/150012, ACRIN 6657. J Clin Oncol 2012; 30: 3242–3249.
Ross RW, Galsky MD, Febbo P, Barry M, Richie JP, Xie W et al. Phase 2 study of neoadjuvant docetaxel plus bevacizumab in patients with high-risk localized prostate cancer: a prostate cancer clinical trials consortium trial. Cancer 2012; 118: 4777–4784.
Partridge SC, Gibbs JE, Lu Y, Esserman LJ, Tripathy D, Wolverton DS et al. MRI measurements of breast tumor volume predict response to neoadjuvant chemotherapy and recurrence-free survival. AJR Am J Roentgenol 2005; 184: 1774–1781.
Antonarakis ES, Feng Z, Trock BJ, Humphreys EB, Carducci MA, Partin AW et al. The natural history of metastatic progression in men with prostate-specific antigen recurrence after radical prostatectomy: long-term follow-up. BJU Int 2012; 109: 32–39.
Hoeks CM, Barentsz JO, Hambrock T, Yakar D, Somford DM, Heijmink SW et al. Prostate cancer: multiparametric MR imaging for detection, localization, and staging. Radiology 2011; 261: 46–66.
Margel D, Yap SA, Lawrentschuk N, Klotz L, Haider M, Hersey K et al. Impact of multiparametric endorectal coil prostate magnetic resonance imaging on disease reclassification among active surveillance candidates: a prospective cohort study. J Urol 2012; 187: 1247–1252.
Turkbey B, Mani H, Shah V, Rastinehad AR, Bernardo M, Pohida T et al. Multiparametric 3 T prostate magnetic resonance imaging to detect cancer: histopathological correlation using prostatectomy specimens processed in customized magnetic resonance imaging based molds. J Urol 2011; 186: 1818–1824.
van As NJ, de Souza NM, Riches SF, Morgan VA, Sohaib SA, Dearnaley DP et al. A study of diffusion-weighted magnetic resonance imaging in men with untreated localised prostate cancer on active surveillance. Eur Urol 2009; 56: 981–987.
Mostaghel EA, Nelson P, Lange PH, Lin D, Taplin M-E, Balk SP et al. Neoadjuvant androgen pathway suppression prior to prostatectomy. ASCO Meeting Abstr 2012; 30: 4520.
Taplin M-E, Montgomery RB, Logothetis C, Bubley GJ, Richie JP, Dalkin BL et al. Effect of neoadjuvant abiraterone acetate (AA) plus leuprolide acetate (LHRHa) on PSA, pathological complete response (pCR), and near pCR in localized high-risk prostate cancer (LHRPC): Results of a randomized phase II study. ASCO Meeting Abstr 2012; 30: 4521.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Galsky served as a consultant to Dendreon, Jannsen, Astellas, Glaxo Smith Kline. Taplin served as a consultant to Sanofi, Medivation, Jannsen, Dendreon, Tokai. The remaining authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Galsky, M., Xie, W., Nakabayashi, M. et al. Analysis of the correlation between endorectal MRI response to neoadjuvant chemotherapy and biochemical recurrence in patients with high-risk localized prostate cancer. Prostate Cancer Prostatic Dis 16, 266–270 (2013). https://doi.org/10.1038/pcan.2013.15
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/pcan.2013.15