Abstract
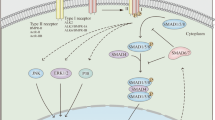
Small mothers against decapentaplegic (SMAD) proteins are a family of signal transduction molecules in transforming growth factor β (TGFβ) ligand pathways that have been found to have a key role in the pathogenesis of inflammatory bowel disease (IBD). Long standing IBD predisposes individuals to colitis-associated colorectal cancer (CAC), an entity that possess unique characteristics compared to hereditary and sporadic cancer. The ligands of the TGFβ super family along with SMADs have also been implicated in several aspects of colorectal cancer formation. SMAD proteins are shown to be involved in a number of potentially carcinogenic mechanisms such as altering gene transcription, controlling stem cell differentiation to causing epigenetic changes. Modulation of these proteins has emerged as a novel therapeutic intervention for IBD although its effect on carcinogenesis remains elusive. This account reviews available evidence linking SMAD proteins to CAC and explores the potential areas for future research in this area.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Virchow R . Cellular pathology.As based upon physiological and pathological histology. J. B. Lippincott: Philadelphia: PA, USA, 1863,. pp 566.
Mantovani A, Allavena P, Sica A, Balkwill F . Cancer-related inflammation. Nature 2008; 454: 436–444.
Bernstein CN, Blanchard JF, Kliewer E, Wajda A . Cancer risk in patients with inflammatory bowel disease: a population-based study. Cancer 2001; 91: 854–862.
Eaden JA, Abrams KR, Mayberry JF . The risk of colorectal cancer in ulcerative colitis: a meta-analysis. Gut 2001; 48: 526–535.
Canavan C, Abrams KR, Mayberry J . Meta-analysis: colorectal and small bowel cancer risk in patients with Crohn’s disease. Aliment Pharmacol Ther 2006; 23: 1097–1104.
Choi C-HR, Rutter MD, Askari A, Lee GH, Warusavitarne J, Moorghen M et al. Forty-year analysis of colonoscopic surveillance program for neoplasia in ulcerative colitis: an updated overview. Am J Gastroenterol 2015; 110: 1022–1034.
Jess T, Horváth-Puhó E, Fallingborg J, Rasmussen HH, Jacobsen BA . Cancer risk in inflammatory bowel disease according to patient phenotype and treatment: a Danish population-based cohort study. Am J Gastroenterol 2013; 108: 1869–1876.
Annese V, Daperno M, Rutter MD, Amiot A, Bossuyt P, East J et al. European evidence based consensus for endoscopy in inflammatory bowel disease ⋆. J Crohn’s Colitis 2013; 7: 982–1018.
Rutter M, Saunders B, Wilkinson K, Rumbles S, Schofield G, Kamm M et al. Severity of inflammation is a risk factor for colorectal neoplasia in ulcerative colitis. Gastroenterology 2004; 126: 451–459.
Hussain SP, Hofseth LJ, Harris CC . Radical causes of cancer. Nat Rev Cancer 2003; 3: 276–285.
Garrity-Park MM, Loftus E V, Sandborn WJ, Bryant SC, Smyrk TC . Methylation status of genes in non-neoplastic mucosa from patients with ulcerative colitis-associated colorectal cancer. Am J Gastroenterol 2010; 105: 1610–1619.
Grivennikov SI . Inflammation and colorectal cancer: colitis-associated neoplasia. Semin Immunopathol 2013; 35: 229–244.
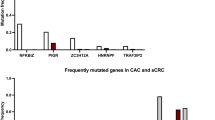
Yaeger R, Shah MA, Miller VA, Kelsen JR, Wang K, Heins ZJ et al. Genomic alterations observed in colitis-associated cancers are distinct from those found in sporadic colorectal cancers and vary by type of inflammatory bowel disease. Gastroenterology 2016; 151: 278–287.e6.
Willenbucher RF, Aust DE, Chang CG, Zelman SJ, Ferrell LD, Moore DH et al. Genomic instability is an early event during the progression pathway of ulcerative-colitis-related neoplasia. Am J Pathol 1999; 154: 1825–1830.
Rhodes JM, Campbell BJ . Inflammation and colorectal cancer: IBD-associated and sporadic cancer compared. Trends Mol Med 2002; 8: 10–16.
Aust DE, Terdiman JP, Willenbucher RF, Chang CG, Molinaro-Clark A, Baretton GB et al. The APC/beta-catenin pathway in ulcerative colitis-related colorectal carcinomas: a mutational analysis. Cancer 2002; 94: 1421–1427.
Brentnall TA, Crispin DA, Rabinovitch PS, Haggitt RC, Rubin CE, Stevens AC et al. Mutations in the p53 gene: an early marker of neoplastic progression in ulcerative colitis. Gastroenterology 1994; 107: 369–378.
Ullman TA, Itzkowitz SH . Intestinal inflammation and cancer. Gastroenterology 2011; 140: 1807–1816.
Sekelsky JJ, Newfeld SJ, Raftery LA, Chartoff EH, Gelbart WM . Genetic characterization and cloning of mothers against dpp, a gene required for decapentaplegic function in Drosophila melanogaster. Genetics 1995; 139: 1347–1358.
Savage C, Das P, Finelli AL, Townsend SR, Sun CY, Baird SE et al. Caenorhabditis elegans genes sma-2, sma-3, and sma-4 define a conserved family of transforming growth factor beta pathway components. Proc Natl Acad Sci USA 1996; 93: 790–794.
Shi Y, Massagué J . Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell 2003; 113: 685–700.
Massagué J . TGFβ signalling in context. Nat Rev Mol Cell Biol 2012; 13: 616–630.
Zhang S, Ekman M, Thakur N, Bu S, Davoodpour P, Grimsby S et al. TGFβ1-induced activation of ATM and p53 mediates apoptosis in a Smad7-dependent manner. Cell Cycle 2006; 5: 2787–2795.
Monteleone G, Kumberova A, Croft NM, McKenzie C, Steer HW, MacDonald TT . Blocking Smad7 restores TGF-beta1 signaling in chronic inflammatory bowel disease. J Clin Invest 2001; 108: 601–609.
Monteleone G, Del Vecchio Blanco G, Monteleone I, Fina D, Caruso R, Gioia V et al. Post-transcriptional regulation of Smad7 in the gut of patients with inflammatory bowel disease. Gastroenterology 2005; 129: 1420–1429.
Babyatsky M, Rossiter G, Podolsky D . Expression of transforming growth factors alpha and beta in colonic mucosa in inflammatory bowel disease. Gastroenterology 1996; 110: 975–984.
Fantini MC, Rizzo A, Fina D, Caruso R, Sarra M, Stolfi C et al. Smad7 controls resistance of colitogenic T cells to regulatory T cell-mediated suppression. Gastroenterology 2009; 136: 1308–1316.e3.
Marafini I, Sedda S, Fusco D, Di, Figliuzzi MM, Pallone F, Monteleone G Smad7 Sustains Inflammation in the Gut: From Bench to Bedside.
Monteleone G, Del G, Blanco V, Monteleone I, Fina D, Caruso R et al. Post-transcriptional regulation of Smad7 in the gut of patients with inflammatory bowel disease. Gastroenterology 2005; 129: 1420–1429.
Boirivant M, Pallone F, Di Giacinto C, Fina D, Monteleone I, Marinaro M et al. Inhibition of Smad7 with a specific antisense oligonucleotide facilitates TGF-β1–mediated suppression of colitis. Gastroenterology 2006; 131: 1786–1798.
Monteleone G, Neurath MF, Ardizzone S, Di Sabatino A, Fantini MC, Castiglione F et al. Mongersen, an oral SMAD7 antisense oligonucleotide, and Crohn’s disease. N Engl J Med 2015; 372: 1104–1113.
Monteleone G, Fantini MC, Onali S, Zorzi F, Sancesario G, Bernardini S et al. Phase I clinical trial of Smad7 knockdown using antisense oligonucleotide in patients with active Crohn’s disease. Mol Ther 2012; 20: 870–876.
Roberts AB, Wakefield LM . The two faces of transforming growth factor in carcinogenesis. Proc Natl Acad Sci 2003; 100: 8621–8623.
Hussain SP, Amstad P, Raja K, Ambs S, Nagashima M, Bennett WP et al. Increased p53 mutation load in noncancerous colon tissue from ulcerative colitis: a cancer-prone chronic inflammatory disease. Cancer Res 2000; 60: 3333–3337.
Atfi A, Baron R . p53 brings a new twist to the Smad signaling network. Sci Signal 2008; 1: pe33.
Cordenonsi M, Dupont S, Maretto S, Insinga A, Imbriano C, Piccolo S . Links between tumor suppressors: p53 is required for TGF-beta gene responses by cooperating with Smads. Cell 2003; 113: 301–314.
Kalo E, Buganim Y, Shapira KE, Besserglick H, Goldfinger N, Weisz L et al. Mutant p53 attenuates the SMAD-dependent transforming growth factor 1 (TGF-1) signaling pathway by repressing the expression of TGF- receptor type II. Mol Cell Biol 2007; 27: 8228–8242.
Elston R, Inman GJ . Crosstalk between p53 and TGF-β Signalling. J Signal Transduct 2012; 2012: 294097.
Qi Z, Li Y, Zhao B, Xu C, Liu Y, Li H et al. BMP restricts stemness of intestinal Lgr5+ stem cells by directly suppressing their signature genes. Nat Commun 2017; 8: 13824.
He XC, Zhang J, Tong W-G, Tawfik O, Ross J, Scoville DH et al. BMP signaling inhibits intestinal stem cell self-renewal through suppression of Wnt–β-catenin signaling. Nat Genet 2004; 36: 1117–1121.
Galvin KE, Travis ED, Yee D, Magnuson T, Vivian JL . Nodal signaling regulates the bone morphogenic protein pluripotency pathway in mouse embryonic stem cells. J Biol Chem 2010; 285: 19747–19756.
Gomes Fernandes M, Dries R, Roost MS, Semrau S, de Melo Bernardo A, Davis RP et al. BMP-SMAD signaling regulates lineage priming, but is dispensable for self-renewal in mouse embryonic stem cells. Stem Cell Rep 2016; 6: 85–94.
Papageorgis P, Lambert AW, Ozturk S, Gao F, Pan H, Manne U et al. Smad signaling is required to maintain epigenetic silencing during breast cancer progression. Cancer Res 2010; 70: 968–978.
Azarschab P, Porschen R, Gregor M, Blin N, Holzmann K . Epigenetic control of the E-cadherin gene (CDH1) by CpG methylation in colectomy samples of patients with ulcerative colitis. Genes Chromosom Cancer 2002; 35: 121–126.
Davis H, Raja E, Miyazono K, Tsubakihara Y, Moustakas A . Mechanisms of action of bone morphogenetic proteins in cancer. Cytokine Growth Factor Rev 2016; 27: 81–92.
Howe JR, Sayed MG, Ahmed AF, Ringold J, Larsen-Haidle J, Merg A et al. The prevalence of MADH4 and BMPR1A mutations in juvenile polyposis and absence of BMPR2, BMPR1B, and ACVR1 mutations. J Med Genet 2004; 41: 484–491.
Kodach LL, Bleuming SA, Musler AR, Peppelenbosch MP, Hommes DW, van den Brink GR et al. The bone morphogenetic protein pathway is active in human colon adenomas and inactivated in colorectal cancer. Cancer 2008; 112: 300–306.
Voorneveld PW, Kodach LL, Jacobs RJ, van Noesel CJM, Peppelenbosch MP, Korkmaz KS et al. The BMP pathway either enhances or inhibits the Wnt pathway depending on the SMAD4 and p53 status in CRC. Br J Cancer 2015; 112: 122–130.
Voorneveld PW, Kodach LL, Jacobs RJ, Liv N, Zonnevylle AC, Hoogenboom JP et al. Loss of SMAD4 alters BMP signaling to promote colorectal cancer cell metastasis via activation of Rho and ROCK. Gastroenterology 2014; 147: 196–208. e13.
Claessen MMH, Schipper MEI, Oldenburg B, Siersema PD, Offerhaus GJA, Vleggaar FP . WNT-pathway activation in IBD-associated colorectal carcinogenesis: potential biomarkers for colonic surveillance. Cell Oncol 2010; 32: 303–310.
Riggins GJ, Kinzler KW, Vogelstein B, Thiagalingam S . Frequency of Smad gene mutations in human cancers. Cancer Res 1997; 57: 2578–2580.
Thompson CL, Plummer SJ, Acheson LS, Tucker TC, Casey G, Li L . Association of common genetic variants in SMAD7 and risk of colon cancer. Carcinogenesis 2009; 30: 982–986.
Slattery ML, Herrick J, Curtin K, Samowitz W, Wolff RK, Caan BJ et al. Increased risk of colon cancer associated with a genetic polymorphism of SMAD7. Cancer Res 2010; 70: 1479–1485.
Damavand B, Derakhshani S, Saeedi N, Mohebbi SR, Milanizadeh S, Azimzadeh P et al. Intronic polymorphisms of the SMAD7 gene in association with colorectal cancer. Asian Pac J Cancer Prev 2015; 16: 41–44.
Huang Y, Wu W, Nie M, Li C, Wang L, Huang Y et al. SMAD7 polymorphisms and colorectal cancer risk: a meta-analysis of case-control studies. Oncotarget 2016; 7: 75561–75570.
Boulay J-L, Mild G, Lowy A, Reuter J, Lagrange M, Terracciano L et al. SMAD7 is a prognostic marker in patients with colorectal cancer. Int J Cancer 2003; 104: 446–449.
Stolfi C, De Simone V, Colantoni A, Franzè E, Ribichini E, Fantini MC et al. A functional role for Smad7 in sustaining colon cancer cell growth and survival. Cell Death Dis 2014; 5: e1073.
De Simone V, Bevivino G, Sedda S, Izzo R, Laudisi F, Dinallo V et al. Smad7 knockdown activates protein kinase RNA-associated eIF2? pathway leading to colon cancer cell death. Cell Death Dis 2017; 8: e2681.
Xie W, Rimm DL, Lin Y, Shih WJ, Reiss M . Loss of Smad signaling in human colorectal cancer is associated with advanced disease and poor prognosis. Cancer J 2003; 9: 302–312.
Massagué J, Hata A, Lo RS, Wotton D, Lagna G . Mutations increasing autoinhibition inactivate tumour suppressors Smad2 and Smad4. Nature 1997; 388: 82–87.
Wang H, Nie L, Wu L, Liu Q, Guo X . NR2F2 inhibits Smad7 expression and promotes TGF-β-dependent epithelial-mesenchymal transition of CRC via transactivation of miR-21. Biochem Biophys Res Commun 2017; 485: 181–188.
Gorelik L, Flavell RA . Immune-mediated eradication of tumors through the blockade of transforming growth factor-beta signaling in T cells. Nat Med 2001; 7: 1118–1122.
Rizzo A, Waldner MJ, Stolfi C, Sarra M, Fina D, Becker C et al. Smad7 expression in T cells prevents colitis-associated cancer. Cancer Res 2011; 71: 7423–7432.
Lu J, Zeng H, Liang Z, Chen L, Zhang L, Zhang H et al. Network modelling reveals the mechanism underlying colitis-associated colon cancer and identifies novel combinatorial anti-cancer targets. Sci Rep 2015; 5: 14739.
Maggio-Price L, Treuting P, Zeng W, Tsang M, Bielefeldt-Ohmann H, Iritani BM . Helicobacter infection is required for inflammation and colon cancer in Smad3-deficient mice. Cancer Res 2006; 66: 828–838.
Stolfi C, Marafini I, De Simone V, Pallone F, Monteleone G . The dual role of Smad7 in the control of cancer growth and metastasis. Int J Mol Sci 2013; Vol. 14: p23774–23790.
Clevers H . Modeling development and disease with organoids. Cell 2016; 165: 1586–1597.
Acknowledgements
We acknowledge the St Mark’s Foundation, ‘Action against cancer’ and the Commonwealth Scholarship commission for their invaluable contribution made towards our research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Chandrasinghe, P., Cereser, B., Moorghen, M. et al. Role of SMAD proteins in colitis-associated cancer: from known to the unknown. Oncogene 37, 1–7 (2018). https://doi.org/10.1038/onc.2017.300
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2017.300
This article is cited by
-
Cellular mechanotransduction in health and diseases: from molecular mechanism to therapeutic targets
Signal Transduction and Targeted Therapy (2023)
-
The Interaction Between SMAD1 and YAP1 Is Correlated with Increased Resistance of Gastric Cancer Cells to Cisplatin
Applied Biochemistry and Biotechnology (2023)
-
From inflammatory bowel disease to colorectal cancer: what’s the role of miRNAs?
Cancer Cell International (2022)
-
The comprehensive detection of miRNA and circRNA in the regulation of intramuscular and subcutaneous adipose tissue of Laiwu pig
Scientific Reports (2022)
-
Inflammatory bowel disease and carcinogenesis
Cancer and Metastasis Reviews (2022)