Abstract
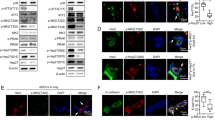
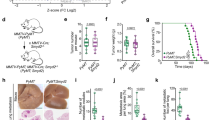
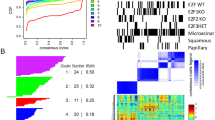
The protein p38 mitogen-activated protein kinase (MAPK) delta isoform (p38δ) is a poorly studied member of the MAPK family. Data analysis from The Cancer Genome Atlas database revealed that p38δ is highly expressed in all types of human breast cancers. Using a human breast cancer tissue array, we confirmed elevation in cancer tissue. The breast cancer mouse model, MMTV-PyMT (PyMT), developed breast tumors with lung metastasis; however, mice deleted in p38δ (PyMT/p38δ−/−) exhibited delayed primary tumor formation and highly reduced lung metastatic burden. At the cellular level, we demonstrate that targeting of p38δ in breast cancer cells, MCF-7 and MDA-MB-231 resulted in a reduced rate of cell proliferation. In addition, cells lacking p38δ also displayed an increased cell–matrix adhesion and reduced cell detachment. This effect on cell adhesion was molecularly supported by the regulation of the focal adhesion kinase by p38δ in the human breast cell lines. These studies define a previously unappreciated role for p38δ in breast cancer development and evolution by regulating tumor growth and altering metastatic properties. This study proposes MAPK p38δ protein as a key factor in breast cancer. Lack of p38δ resulted in reduced primary tumor size and blocked the metastatic potential to the lungs.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Cuadrado A, Nebreda AR . Mechanisms and functions of p38 MAPK signalling. Biochem J 2010; 429: 403–417.
O'Callaghan C, Fanning LJ, Barry OP . p38delta MAPK: emerging roles of a neglected isoform. Int J Cell Biol 2014; 2014: 272689.
Sabio G, Arthur JS, Kuma Y, Peggie M, Carr J, Murray-Tait V et al. p38gamma regulates the localisation of SAP97 in the cytoskeleton by modulating its interaction with GKAP. EMBO J 2005; 24: 1134–1145.
Ittner A, Block H, Reichel CA, Varjosalo M, Gehart H, Sumara G et al. Regulation of PTEN activity by p38delta-PKD1 signaling in neutrophils confers inflammatory responses in the lung. J Exp Med 2012; 209: 2229–2246.
Risco A, del Fresno C, Mambol A, Alsina-Beauchamp D, MacKenzie KF, Yang HT et al. p38gamma and p38delta kinases regulate the Toll-like receptor 4 (TLR4)-induced cytokine production by controlling ERK1/2 protein kinase pathway activation. Proc Natl Acad Sci USA 2012; 109: 11200–11205.
O'Callaghan C, Fanning LJ, Houston A, Barry OP . Loss of p38delta mitogen-activated protein kinase expression promotes oesophageal squamous cell carcinoma proliferation, migration and anchorage-independent growth. Int J Oncol 2013; 43: 405–415.
Junttila MR, Ala-Aho R, Jokilehto T, Peltonen J, Kallajoki M, Grenman R et al. p38alpha and p38delta mitogen-activated protein kinase isoforms regulate invasion and growth of head and neck squamous carcinoma cells. Oncogene 2007; 26: 5267–5279.
Tan FL, Ooi A, Huang D, Wong JC, Qian CN, Chao C et al. p38delta/MAPK13 as a diagnostic marker for cholangiocarcinoma and its involvement in cell motility and invasion. Int J Cancer 2010; 126: 2353–2361.
Zhong J, Lardinois D, Szilard J, Tamm M, Roth M . Rat mesothelioma cell proliferation requires p38delta mitogen activated protein kinase and C/EBP-alpha. Lung Cancer 2011; 73: 166–170.
Schindler EM, Hindes A, Gribben EL, Burns CJ, Yin Y, Lin MH et al. p38delta Mitogen-activated protein kinase is essential for skin tumor development in mice. Cancer Res 2009; 69: 4648–4655.
Del Reino P, Alsina-Beauchamp D, Escos A, Cerezo-Guisado MI, Risco A, Aparicio N et al. Pro-oncogenic role of alternative p38 mitogen-activated protein kinases p38gamma and p38delta, linking inflammation and cancer in colitis-associated colon cancer. Cancer Res 2014; 74: 6150–6160.
Chen L, Mayer JA, Krisko TI, Speers CW, Wang T, Hilsenbeck SG et al. Inhibition of the p38 kinase suppresses the proliferation of human ER-negative breast cancer cells. Cancer Res 2009; 69: 8853–8861.
Siegel RL, Miller KD, Jemal A . Cancer Statistics, 2017. CA Cancer J Clin 2017; 67: 7–30.
Kohler BA, Sherman RL, Howlader N, Jemal A, Ryerson AB, Henry KA et al. Annual Report to the Nation on the Status of Cancer, 1975-2011, featuring incidence of breast cancer subtypes by race/ethnicity, poverty, and state. J Natl Cancer Inst 2015; 107: djv048.
Kesson EM, Allardice GM, George WD, Burns HJ, Morrison DS . Effects of multidisciplinary team working on breast cancer survival: retrospective, comparative, interventional cohort study of 13 722 women. BMJ 2012; 344: e2718.
Kitatani K, Sheldon K, Anelli V, Jenkins RW, Sun Y, Grabowski GA et al. Acid beta-glucosidase 1 counteracts p38delta-dependent induction of interleukin-6: possible role for ceramide as an anti-inflammatory lipid. J Biol Chem 2009; 284: 12979–12988.
Dethlefsen C, Hojfeldt G, Hojman P . The role of intratumoral and systemic IL-6 in breast cancer. Breast Cancer Res Treat 2013; 138: 657–664.
Choi YK, Woo SM, Cho SG, Moon HE, Yun YJ, Kim JW et al. Brain-metastatic triple-negative breast cancer cells regain growth ability by altering gene expression patterns. Cancer Genomics Proteomics 2013; 10: 265–275.
Guy CT, Cardiff RD, Muller WJ . Induction of mammary tumors by expression of polyomavirus middle T oncogene: a transgenic mouse model for metastatic disease. Mol Cell Biol 1992; 12: 954–961.
Liu Y, Cao W, Zhang B, Liu YQ, Wang ZY, Wu YP et al. The natural compound magnolol inhibits invasion and exhibits potential in human breast cancer therapy. Sci Rep 2013; 3: 3098.
Luo M, Guan JL . Focal adhesion kinase: a prominent determinant in breast cancer initiation, progression and metastasis. Cancer Lett 2010; 289: 127–139.
Sulzmaier FJ, Jean C, Schlaepfer DD . FAK in cancer: mechanistic findings and clinical applications. Nat Rev Cancer 2014; 14: 598–610.
Aust S, Auer K, Bachmayr-Heyda A, Denkert C, Sehouli J, Braicu I et al. Ambivalent role of pFAK-Y397 in serous ovarian cancer—a study of the OVCAD consortium. Mol Cancer 2014; 13: 67.
Owens LV, Xu L, Craven RJ, Dent GA, Weiner TM, Kornberg L et al. Overexpression of the focal adhesion kinase (p125FAK) in invasive human tumors. Cancer Res 1995; 55: 2752–2755.
Lahlou H, Sanguin-Gendreau V, Zuo D, Cardiff RD, McLean GW, Frame MC et al. Mammary epithelial-specific disruption of the focal adhesion kinase blocks mammary tumor progression. Proc Natl Acad Sci USA 2007; 104: 20302–20307.
Provenzano PP, Inman DR, Eliceiri KW, Beggs HE, Keely PJ . Mammary epithelial-specific disruption of focal adhesion kinase retards tumor formation and metastasis in a transgenic mouse model of human breast cancer. Am J Pathol 2008; 173: 1551–1565.
Luo M, Fan H, Nagy T, Wei H, Wang C, Liu S et al. Mammary epithelial-specific ablation of the focal adhesion kinase suppresses mammary tumorigenesis by affecting mammary cancer stem/progenitor cells. Cancer Res 2009; 69: 466–474.
Pylayeva Y, Gillen KM, Gerald W, Beggs HE, Reichardt LF, Giancotti FG . Ras- and PI3K-dependent breast tumorigenesis in mice and humans requires focal adhesion kinase signaling. J Clin Invest 2009; 119: 252–266.
Arold ST . How focal adhesion kinase achieves regulation by linking ligand binding, localization and action. Curr Opin Struct Biol 2011; 21: 808–813.
Fluck MM, Schaffhausen BS . Lessons in signaling and tumorigenesis from polyomavirus middle T antigen. Microbiol Mol Biol Rev 2009; 73: 542–563.
Jones LM, Broz ML, Ranger JJ, Ozcelik J, Ahn R, Zuo D et al. STAT3 establishes an immunosuppressive microenvironment during the early stages of breast carcinogenesis to promote tumor growth and metastasis. Cancer Res. 2015; 76: 1416–1428.
Place AE, Jin Huh S, Polyak K . The microenvironment in breast cancer progression: biology and implications for treatment. Breast Cancer Res 2011; 13: 227.
McAllister SS, Weinberg RA . The tumour-induced systemic environment as a critical regulator of cancer progression and metastasis. Nat Cell Biol 2014; 16: 717–727.
Debnath J, Muthuswamy SK, Brugge JS . Morphogenesis and oncogenesis of MCF-10A mammary epithelial acini grown in three-dimensional basement membrane cultures. Methods 2003; 30: 256–268.
Adada MM, Canals D, Jeong N, Kelkar AD, Hernandez-Corbacho M, Pulkoski-Gross MJ et al. Intracellular sphingosine kinase 2-derived sphingosine-1-phosphate mediates epidermal growth factor-induced ezrin-radixin-moesin phosphorylation and cancer cell invasion. FASEB J 2015; 29: 4654–4669.
Orr Gandy KA, Adada M, Canals D, Carroll B, Roddy P, Hannun YA et al. Epidermal growth factor-induced cellular invasion requires sphingosine-1-phosphate/sphingosine-1-phosphate 2 receptor-mediated ezrin activation. FASEB J 2013; 27: 3155–3166.
Acknowledgements
We thank Dr Eiji Suzuki (Fukushima Medical University), Dr Tatiana Efimova (Washington University), Dr Patricia Watson, Dr Dennis K. Watson and George Washington (Medical University of South Carolina), Dr Hideki Furuya, Dr Toshihiko Kawamori (University of Hawaii), Dr Vincent Yang and members of his laboratory, Mallory Korman, Dr Luisa Escobar-Hoyos, Dr Kai Wang, Dr Chiara Luberto, Dr Achraf Shamsedine, Dr Mónica Garcia-Barros, Dr Jean-Philip Truman, Maria Hernandez, Dr Ashley Snider and Dr Magali Trayssac (Stony Brook University) for helpful technical advice and/or assistance. This work is supported by the National Cancer Institute (R01-CA87584). These studies were supported by NIH Grant CA087584.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Rights and permissions
About this article
Cite this article
Wada, M., Canals, D., Adada, M. et al. P38 delta MAPK promotes breast cancer progression and lung metastasis by enhancing cell proliferation and cell detachment. Oncogene 36, 6649–6657 (2017). https://doi.org/10.1038/onc.2017.274
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2017.274
This article is cited by
-
The genomic regulation of metastatic dormancy
Cancer and Metastasis Reviews (2023)
-
Analysis and validation of m6A regulatory network: a novel circBACH2/has-miR-944/HNRNPC axis in breast cancer progression
Journal of Translational Medicine (2021)
-
MAGI1 inhibits the AMOTL2/p38 stress pathway and prevents luminal breast tumorigenesis
Scientific Reports (2021)
-
Breast cancer dormancy: need for clinically relevant models to address current gaps in knowledge
npj Breast Cancer (2021)
-
RETRACTED ARTICLE: MicroRNA-4268 inhibits cell proliferation via AKT/JNK signalling pathways by targeting Rab6B in human gastric cancer
Cancer Gene Therapy (2020)