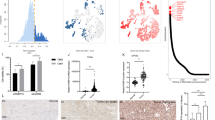
Abstract
Targeted therapeutics, such as those abrogating hypoxia inducible factor (HIF)/vascular endothelial growth factor signaling, are initially effective against kidney cancer (or renal cell carcinoma, RCC); however, drug resistance frequently occurs via subsequent activation of alternative pathways. Through genome-scale integrated analysis of the HIF-α network, we identified the major protein kinase C substrate MARCKS (myristoylated alanine-rich C kinase substrate) as a potential target molecule for kidney cancer. In a screen of nephrectomy samples from 56 patients with RCC, we found that MARCKS expression and its phosphorylation are increased and positively correlate with tumor grade. Genetic and pharmacologic suppression of MARCKS in high-grade RCC cell lines in vitro led to a decrease in cell proliferation and migration. We further demonstrated that higher MARCKS expression promotes growth and angiogenesis in vivo in an RCC xenograft tumor. MARCKS acted upstream of the AKT/mTOR pathway, activating HIF-target genes, notably vascular endothelial growth factor-A. Following knockdown of MARCKS in RCC cells, the IC50 of the multikinase inhibitor regorafenib was reduced. Surprisingly, attenuation of MARCKS using the MPS (MARCKS phosphorylation site domain) peptide synergistically interacted with regorafenib treatment and decreased survival of kidney cancer cells through inactivation of AKT and mTOR. Our data suggest a major contribution of MARCKS to kidney cancer growth and provide an alternative therapeutic strategy of improving the efficacy of multikinase inhibitors.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
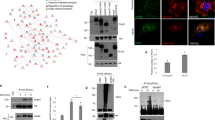
References
Cancer Genome Atlas Research N. Comprehensive molecular characterization of clear cell renal cell carcinoma. Nature 2013; 499: 43–49.
Cowey CL, Rathmell WK . VHL gene mutations in renal cell carcinoma: role as a biomarker of disease outcome and drug efficacy. Curr Oncol Rep 2009; 11: 94–101.
Masoud GN, Li W . HIF-1alpha pathway: role, regulation and intervention for cancer therapy. Acta Pharm Sin B 2015; 5: 378–389.
Gudas LJ, Fu L, Minton DR, Mongan NP, Nanus DM . The role of HIF1alpha in renal cell carcinoma tumorigenesis. J Mol Med 2014; 92: 825–836.
Domblides C, Gross-Goupil M, Quivy A, Ravaud A . Emerging antiangiogenics for renal cancer. Expert Opin Emerg Drugs 2013; 18: 495–511.
Su D, Stamatakis L, Singer EA, Srinivasan R . Renal cell carcinoma: molecular biology and targeted therapy. Curr Opin Oncol 2014; 26: 321–327.
Joosten SC, Hamming L, Soetekouw PM, Aarts MJ, Veeck J, van Engeland M et al. Resistance to sunitinib in renal cell carcinoma: From molecular mechanisms to predictive markers and future perspectives. Biochim Biophys Acta 2015; 1855: 1–16.
Zhou L, Liu XD, Sun M, Zhang X, German P, Bai S et al. Targeting MET and AXL overcomes resistance to sunitinib therapy in renal cell carcinoma. Oncogene 2016; 35: 2687–2697.
Santoni M, Pantano F, Amantini C, Nabissi M, Conti A, Burattini L et al. Emerging strategies to overcome the resistance to current mTOR inhibitors in renal cell carcinoma. Biochim Biophys Acta 2014; 1845: 221–231.
Gross-Goupil M, Massard C, Ravaud A . Targeted therapies in metastatic renal cell carcinoma: overview of the past year. Curr Urol Rep 2012; 13: 16–23.
Parekh H, Rini BI . Emerging therapeutic approaches in renal cell carcinoma. Expert Rev Anticancer Ther 2015; 15: 1305–1314.
Dorff TB, Pal SK, Quinn DI . Novel tyrosine kinase inhibitors for renal cell carcinoma. Expert Rev Clin Pharmacol 2014; 7: 67–73.
Wilhelm SM, Dumas J, Adnane L, Lynch M, Carter CA, Schütz G et al. Regorafenib (BAY 73-4506): a new oral multikinase inhibitor of angiogenic, stromal and oncogenic receptor tyrosine kinases with potent preclinical antitumor activity. Int J Cancer 2011; 129: 245–255.
Sajithlal GB, Hamed HA, Cruickshanks N, Booth L, Tavallai S, Syed J et al. Sorafenib/regorafenib and phosphatidyl inositol 3 kinase/thymoma viral proto-oncogene inhibition interact to kill tumor cells. Mol Pharmacol 2013; 84: 562–571.
Eisen T, Joensuu H, Nathan PD, Harper PG, Wojtukiewicz MZ, Nicholson S et al. Regorafenib for patients with previously untreated metastatic or unresectable renal-cell carcinoma: a single-group phase 2 trial. Lancet Oncol 2012; 13: 1055–1062.
Zaki K, Aslam S, Eisen T . Regorafenib (BAY 73-4506): stromal and oncogenic multikinase inhibitor with potential activity in renal cell carcinoma. Curr Oncol Rep 2013; 15: 91–97.
Aderem A . Signal transduction and the actin cytoskeleton: the roles of MARCKS and profilin. Trends Biochem Sci 1992; 17: 438–443.
Chen X, Rotenberg SA . PhosphoMARCKS drives motility of mouse melanoma cells. Cell Signal 2010; 22: 1097–1103.
Micallef J, Taccone M, Mukherjee J, Croul S, Busby J, Moran MF et al. Epidermal growth factor receptor variant III-induced glioma invasion is mediated through myristoylated alanine-rich protein kinase C substrate overexpression. Cancer Res 2009; 69: 7548–7556.
Jarboe JS, Anderson JC, Duarte CW, Mehta T, Nowsheen S, Hicks PH et al. MARCKS regulates growth and radiation sensitivity and is a novel prognostic factor for glioma. Clin Cancer Res 2012; 18: 3030–3041.
Brooks G, Brooks SF, Goss MW . MARCKS functions as a novel growth suppressor in cells of melanocyte origin. Carcinogenesis 1996; 17: 683–689.
Chen CH, Cheng CT, Yuan Y, Zhai J, Arif M, Fong LW et al. Elevated MARCKS phosphorylation contributes to unresponsiveness of breast cancer to paclitaxel treatment. Oncotarget 2015; 6: 15194–15208.
Chen CH, Statt S, Chiu CL, Zhai J, Arif M, Fong LW et al. Targeting myristoylated alanine-rich C kinase substrate phosphorylation site domain in lung cancer. Mechanisms and therapeutic implications. Am J Respir Crit Care Med 2014; 190: 1127–1138.
Rombouts K, Carloni V, Mello T, Omenetti S, Galastri S, Madiai S et al. Myristoylated alanine-rich protein kinase C substrate (MARCKS) expression modulates the metastatic phenotype in human and murine colon carcinoma in vitro and in vivo. Cancer Lett 2013; 333: 244–252.
Yang Y, Chen Y, Saha MN, Chen J, Evans K, Qiu L et al. Targeting phospho-MARCKS overcomes drug-resistance and induces antitumor activity in preclinical models of multiple myeloma. Leukemia 2014; 29 (3): 715–726.
Browne BC, Hochgrafe F, Wu J, Millar EK, Barraclough J, Stone A et al. Global characterization of signalling networks associated with tamoxifen resistance in breast cancer. The FEBS journal 2013; 280: 5237–5257.
Chen CH, Thai P, Yoneda K, Adler KB, Yang PC, Wu R . A peptide that inhibits function of Myristoylated Alanine-Rich C Kinase Substrate (MARCKS) reduces lung cancer metastasis. Oncogene 2014; 33: 3696–3706.
Chen CH, Chiu CL, Adler KB, Wu R . A novel predictor of cancer malignancy: up-regulation of myristoylated alanine-rich C kinase substrate phosphorylation in lung cancer. Am J Respir Crit Care Med 2014; 189: 1002–1004.
Hanada S, Kakehashi A, Nishiyama N, Wei M, Yamano S, Chung K et al. Myristoylated alanine-rich C-kinase substrate as a prognostic biomarker in human primary lung squamous cell carcinoma. Cancer Biomark 2013; 13: 289–298.
Naboulsi W, Megger DA, Bracht T, Kohl M, Turewicz M, Eisenacher M et al. Quantitative tissue proteomics analysis reveals versican as potential biomarker for early-stage hepatocellular carcinoma. J Proteome Res 2016; 15: 38–47.
Brandi J, Pozza ED, Dando I, Biondani G, Robotti E, Jenkins R et al. Secretome protein signature of human pancreatic cancer stem-like cells. J Proteomics 2016; 136: 1–12.
Bickeboller M, Tagscherer KE, Kloor M, Jansen L, Chang-Claude J, Brenner H et al. Functional characterization of the tumor-suppressor MARCKS in colorectal cancer and its association with survival. Oncogene 2015; 34: 1150–1159.
Greene CS, Krishnan A, Wong AK, Ricciotti E, Zelaya RA, Himmelstein DS et al. Understanding multicellular function and disease with human tissue-specific networks. Nat Genet 2015; 47: 569–576.
Josic D, Clifton JG, Kovac S, Hixson DC . Membrane proteins as diagnostic biomarkers and targets for new therapies. Curr Opin Mol Ther 2008; 10: 116–123.
von Roemeling CA, Radisky DC, Marlow LA, Cooper SJ, Grebe SK, Anastasiadis PZ et al. Neuronal pentraxin 2 supports clear cell renal cell carcinoma by activating the AMPA-selective glutamate receptor-4. Cancer Res 2014; 74: 4796–4810.
Wettersten HI, Hakimi AA, Morin D, Bianchi C, Johnstone ME, Donohoe DR et al. Grade-dependent metabolic reprogramming in kidney cancer revealed by combined proteomics and metabolomics analysis. Cancer Res 2015; 75: 2541–2552.
Hanahan D, Weinberg RA . Hallmarks of cancer: the next generation. Cell 2011; 144: 646–674.
Posadas EM, Limvorasak S, Sharma S, Figlin RA . Targeting angiogenesis in renal cell carcinoma. Expert Opin Pharmacother 2013; 14: 2221–2236.
Maroto P, Rini B . Molecular biomarkers in advanced renal cell carcinoma. Clin Cancer Res 2014; 20: 2060–2071.
Koh MY, Lemos R Jr., Liu X, Powis G . The hypoxia-associated factor switches cells from HIF-1alpha- to HIF-2alpha-dependent signaling promoting stem cell characteristics, aggressive tumor growth and invasion. Cancer Res 2011; 71: 4015–4027.
Sourbier C, Lindner V, Lang H, Agouni A, Schordan E, Danilin S et al. The phosphoinositide 3-kinase/Akt pathway: a new target in human renal cell carcinoma therapy. Cancer Res 2006; 66: 5130–5142.
Ziemba BP, Burke JE, Masson G, Williams RL, Falke JJ . Regulation of PI3K by PKC and MARCKS: single-molecule analysis of a reconstituted signaling pathway. Biophys J 2016; 110: 1811–1825.
Kalwa H, Michel T . The MARCKS protein plays a critical role in phosphatidylinositol 4,5-bisphosphate metabolism and directed cell movement in vascular endothelial cells. J Biol Chem 2011; 286: 2320–2330.
Chou TC, Talalay P . Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul 1984; 22: 27–55.
Han KS, Raven PA, Frees S, Gust K, Fazli L, Ettinger S et al. Cellular adaptation to VEGF-targeted antiangiogenic therapy induces evasive resistance by overproduction of alternative endothelial cell growth factors in renal cell carcinoma. Neoplasia 2015; 17: 805–816.
Kaelin WG Jr . The von Hippel-Lindau tumor suppressor gene and kidney cancer. Clin Cancer Res 2004; 10: 6290S–6295S.
Choueiri TK, Fay AP, Gagnon R, Lin Y, Bahamon B, Brown V et al. The role of aberrant VHL/HIF pathway elements in predicting clinical outcome to pazopanib therapy in patients with metastatic clear-cell renal cell carcinoma. Clin Cancer Res 2013; 19: 5218–5226.
Karar J, Maity A . PI3K/AKT/mTOR pathway in angiogenesis. Front Mol Neurosci 2011; 4: 51.
Zhou J, Schmid T, Frank R, Brune B . PI3K/Akt is required for heat shock proteins to protect hypoxia-inducible factor 1alpha from pVHL-independent degradation. J Biol Chem 2004; 279: 13506–13513.
Ferraro D, Zalcberg J . Regorafenib in gastrointestinal stromal tumors: clinical evidence and place in therapy. Ther Adv Med Oncol 2014; 6: 222–228.
Rauch ME, Ferguson CG, Prestwich GD, Cafiso DS . Myristoylated alanine-rich C kinase substrate (MARCKS) sequesters spin-labeled phosphatidylinositol 4,5-bisphosphate in lipid bilayers. J Biol Chem 2002; 277: 14068–14076.
Rohrbach TD, Shah N, Jackson WP, Feeney EV, Scanlon S, Gish R et al. The effector domain of MARCKS is a nuclear localization signal that regulates cellular PIP2 levels and nuclear PIP2 localization. PloS One 2015; 10: e0140870.
Acknowledgements
The authors thank Mr Muhammad S Arif (Department of Public Health Sciences, University of California at Davis, Davis, CA, USA) for assistance with the experiments; Dr Yu-Ching Lin (UNIMED Healthcare Inc., Taiwan) and Ms Wen-Hsin Chang (Institute of Molecular Medicine, National Taiwan University College of Medicine, Taipei, Taiwan) for technological support in ForteBio system; Dr Guan-Chin Tseng (Department of Pathology, China Medical University Hospital, Taiwan) for useful advice; and the UC Davis Comprehensive Cancer Center Biorepository (University of California at Davis, Davis, CA, USA) for pathology support. This work was supported by NIH grants 1R01CA135401-01A1, 1R03CA181837-01 and 1R01DK082690-01A1, the Medical Service of the US Department of Veterans’ Affairs (all to RHW), and a research grant from Dialysis Clinic, Inc. (DCI# C-3917 to C-HC).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Chen, CH., Fong, L., Yu, E. et al. Upregulation of MARCKS in kidney cancer and its potential as a therapeutic target. Oncogene 36, 3588–3598 (2017). https://doi.org/10.1038/onc.2016.510
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2016.510
This article is cited by
-
Integrated single-cell RNA sequencing analysis reveals a mesenchymal stem cell-associated signature for estimating prognosis and drug sensitivity in gastric cancer
Journal of Cancer Research and Clinical Oncology (2023)
-
Antler-derived microRNA PC-5p-1090 inhibits HCC cell proliferation, migration, and invasion by targeting MARCKS, SMARCAD1, and SOX9
Functional & Integrative Genomics (2023)
-
Pathophysiological roles of myristoylated alanine-rich C-kinase substrate (MARCKS) in hematological malignancies
Biomarker Research (2021)
-
Longitudinal single-cell profiling reveals molecular heterogeneity and tumor-immune evolution in refractory mantle cell lymphoma
Nature Communications (2021)
-
A cell-penetrating MARCKS mimetic selectively triggers cytolytic death in glioblastoma
Oncogene (2020)