Abstract
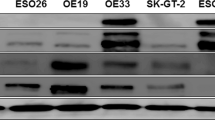
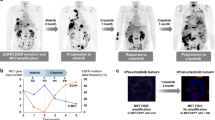
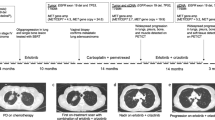
Amplification of the MET oncogene occurs in 2–4% of gastroesophageal cancers and defines a small and aggressive subset of tumors. Although in vitro studies have given very promising results, clinical trials with MET inhibitors have been disappointing, showing few and short lasting responses. The aim of the work was to exploit a MET-amplified patient-derived xenograft model to optimize anti-MET therapeutic strategies in gastroesophageal cancer. We found that despite the high MET amplification level (26 gene copies), in the absence of qualitative or quantitative alterations of EGFR, MET inhibitors induced only tumor growth inhibition, whereas dual MET/EGFR inhibition led to complete tumor regression. Importantly, the combo treatment completely prevented the onset of resistance, which quite rapidly appeared in tumors treated with MET monotherapy. We found that this secondary resistance was due to EGFR activation and could be overcome by dual MET/EGFR inhibition. Similar results were also obtained in a MET-addicted, established gastric cancer cell line. In vitro experiments performed on tumor-derived primary cells confirmed that MET inhibitors were not able to abrogate the activation of downstream transducers and that only the combined MET/EGFR treatment completely shut off the signaling. Previously reported cases, as well as those described here, showed only partial and transient sensitivity to anti-MET therapy. The finding that combined anti-MET/EGFR therapy—even in the absence of EGFR genetic alterations—induced complete and durable response, represents a proof of concept and guarantees further investigations, opening a new perspective of treatment for these patients.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Crew KD, Neugut AI . Epidemiology of upper gastrointestinal malignancies. Semin Oncol 2004; 31: 450–464.
Wild CP, Hardie LJ . Reflux, Barrett's oesophagus and adenocarcinoma: burning questions. Nat Rev Cancer 2003; 3: 676–684.
Network CGAR. Comprehensive molecular characterization of gastric adenocarcinoma. Nature 2014; 513: 202–209.
Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 2010; 376: 687–697.
Comoglio PM, Giordano S, Trusolino L . Drug development of MET inhibitors: targeting oncogene addiction and expedience. Nat Rev Drug Discov 2008; 7: 504–516.
Shah MA, Wainberg ZA, Catenacci DV, Hochster HS, Ford J, Kunz P et al. Phase II study evaluating 2 dosing schedules of oral foretinib (GSK1363089), cMET/VEGFR2 inhibitor, in patients with metastatic gastric cancer. PLoS One 2013; 8: e54014.
Lennerz JK, Kwak EL, Ackerman A, Michael M, Fox SB, Bergethon K et al. MET amplification identifies a small and aggressive subgroup of esophagogastric adenocarcinoma with evidence of responsiveness to crizotinib. J Clin Oncol 2011; 29: 4803–4810.
Shah AM, Bang Y-J, Lordick F, Tabernero J, Chen M, Hack SP et al. METGastric: a phase III study of onartuzumab plus mFOLFOX6 in patients with metastatic HER2-negative (HER2-) and MET-positive (MET+) adenocarcinoma of the stomach or gastroesophageal junction (GEC). J Clin Oncol 2015; 33: (abstract 4012).
Doi T, Kang Y-K, Muro K, Jiang Y, Jain KR, Lizambri R . A phase 3, multicenter, randomized, double-blind, placebo-controlled study of rilotumumab in combination with cisplatin and capecitabine (CX) as first-line therapy for Asian patients (pts) with advanced MET-positive gastric or gastroesophageal junction (G/GEJ) adenocarcinoma: The RILOMET-2 trial. J Clin Oncol 2015; 33 (suppl 3): (abstract TPS226).
Cunningham D, Tebbutt CN, Davidenko I, Murad MA, Al-Batran S-E,., Ilson HD et al. Phase III, randomized, double-blind, multicenter, placebo (P)-controlled trial of rilotumumab (R) plus epirubicin, cisplatin and capecitabine (ECX) as first-line therapy in patients (pts) with advanced MET-positive (pos) gastric or gastroesophageal junction (G/GEJ) cancer: RILOMET-1 study. J Clin Oncol 2015; 33: (abstract 4000).
Hidalgo M, Amant F, Biankin AV, Budinská E, Byrne AT, Caldas C et al. Patient-derived xenograft models: an emerging platform for translational cancer research. Cancer Discov 2014; 4: 998–1013.
Gao H, Korn JM, Ferretti S, Monahan JE, Wang Y, Singh M et al. High-throughput screening using patient-derived tumor xenografts to predict clinical trial drug response. Nat Med 2015; 21: 1318–1325.
Smolen GA, Sordella R, Muir B, Mohapatra G, Barmettler A, Archibald H et al. Amplification of MET may identify a subset of cancers with extreme sensitivity to the selective tyrosine kinase inhibitor PHA-665752. Proc Natl Acad Sci USA 2006; 103: 2316–2321.
Corso S, Ghiso E, Cepero V, Sierra JR, Migliore C, Bertotti A et al. Activation of HER family members in gastric carcinoma cells mediates resistance to MET inhibition. Mol Cancer 2010; 9: 121.
Lutterbach B, Zeng Q, Davis LJ, Hatch H, Hang G, Kohl NE et al. Lung cancer cell lines harboring MET gene amplification are dependent on Met for growth and survival. Cancer Res 2007; 67: 2081–2088.
Comoglio PM, Trusolino L . Invasive growth: from development to metastasis. J Clin Invest 2002; 109: 857–862.
Birchmeier C, Birchmeier W, Gherardi E, Vande Woude GF . Met metastasis, motility and more. Nat Rev Mol Cell Biol 2003; 4: 915–925.
Corso S, Migliore C, Ghiso E, De Rosa G, Comoglio PM, Giordano S . Silencing the MET oncogene leads to regression of experimental tumors and metastases. Oncogene 2008; 27: 684–693.
Peters S, Adjei AA . MET: a promising anticancer therapeutic target. Nat Rev Clin Oncol 2012; 9: 314–326.
Paik PK, Drilon A, Fan PD, Yu H, Rekhtman N, Ginsberg MS et al. Response to MET inhibitors in patients with stage IV lung adenocarcinomas harboring MET mutations causing exon 14 skipping. Cancer Discov 2015; 5: 842–849.
Frampton GM, Ali SM, Rosenzweig M, Chmielecki J, Lu X, Bauer TM et al. Activation of MET via diverse exon 14 splicing alterations occurs in multiple tumor types and confers clinical sensitivity to MET inhibitors. Cancer Discov 2015; 5: 850–859.
Lee J, Ou SH, Lee JM, Kim HC, Hong M, Kim SY et al. Gastrointestinal malignancies harbor actionable MET exon 14 deletions. Oncotarget 2015; 6: 28211–28222.
Cepero V, Sierra JR, Corso S, Ghiso E, Casorzo L, Perera T et al. MET and KRAS gene amplification mediates acquired resistance to MET tyrosine kinase inhibitors. Cancer Res 2010; 70: 7580–7590.
Qi J, McTigue MA, Rogers A, Lifshits E, Christensen JG, Jänne PA et al. Multiple mutations and bypass mechanisms can contribute to development of acquired resistance to MET inhibitors. Cancer Res 2011; 71: 1081–1091.
Bachleitner-Hofmann T, Sun MY, Chen CT, Tang L, Song L, Zeng Z et al. HER kinase activation confers resistance to MET tyrosine kinase inhibition in MET oncogene-addicted gastric cancer cells. Mol Cancer Ther 2008; 7: 3499–3508.
Corso S, Comoglio PM, Giordano S . Cancer therapy: can the challenge be MET? Trends Mol Med 2005; 11: 284–292.
Puri N, Salgia R . Synergism of EGFR and c-Met pathways, cross-talk and inhibition, in non-small cell lung cancer. J Carcinog 2008; 7: 9.
Breindel JL, Haskins JW, Cowell EP, Zhao M, Nguyen DX, Stern DF . EGF receptor activates MET through MAPK to enhance non-small cell lung carcinoma invasion and brain metastasis. Cancer Res 2013; 73: 5053–5065.
Guo A, Villén J, Kornhauser J, Lee KA, Stokes MP, Rikova K et al. Signaling networks assembled by oncogenic EGFR and c-Met. Proc Natl Acad Sci USA 2008; 105: 692–697.
Engelman JA, Zejnullahu K, Mitsudomi T, Song Y, Hyland C, Park JO et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science 2007; 316: 1039–1043.
Bean J, Brennan C, Shih JY, Riely G, Viale A, Wang L et al. MET amplification occurs with or without T790M mutations in EGFR mutant lung tumors with acquired resistance to gefitinib or erlotinib. Proc Natl Acad Sci USA 2007; 104: 20932–20937.
Bertotti A, Burbridge MF, Gastaldi S, Galimi F, Torti D, Medico E et al. Only a subset of Met-activated pathways are required to sustain oncogene addiction. Sci Signal 2009; 2: er11.
Bertotti A, Migliardi G, Galimi F, Sassi F, Torti D, Isella C et al. A molecularly annotated platform of patient-derived xenografts (‘xenopatients’) identifies HER2 as an effective therapeutic target in cetuximab-resistant colorectal cancer. Cancer Discov 2011; 1: 508–523.
Acknowledgements
We thank all our colleagues for helpful scientific discussion; Drs Martinoglio, Porporato and Buscarino for technical support with RT–PCR and sequencing; Dr Perra for tumor microdissection; Dr Giove for support with immunohistochemistry; animal facility employees; Dr Natale for critical manuscript reading. SG and SC are EuroPDX Consortium members. This work was funded by the Italian Association for Cancer Research (AIRC); IG grant 15464 to SG and Fondazione Piemontese per la Ricerca sul Cancro (ONLUS) 5 X 1000 Fondi Ministero della Salute 2013.
Author contributions
SG and SC conceived the study; SG, SC, MA, S Marsoni and PMC contributed to design the experiments; MDS provided the patient material, MA, CM, TC, S Menegon and MC performed experiments; MD contributed to mouse surgery; A Sapino, PC, IS performed the pathologic analysis; LC performed FISH analysis; A Sottile performed the blood biochemical analyses; SG, SC and MA wrote the manuscript. All authors revised the manuscript.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Apicella, M., Migliore, C., Capelôa, T. et al. Dual MET/EGFR therapy leads to complete response and resistance prevention in a MET-amplified gastroesophageal xenopatient cohort. Oncogene 36, 1200–1210 (2017). https://doi.org/10.1038/onc.2016.283
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2016.283
This article is cited by
-
Fatty acid synthase as a new therapeutic target for HER2-positive gastric cancer
Cellular Oncology (2023)
-
Efficacy of CAR-T immunotherapy in MET overexpressing tumors not eligible for anti-MET targeted therapy
Journal of Experimental & Clinical Cancer Research (2022)
-
hOA-DN30: a highly effective humanized single-arm MET antibody inducing remission of ‘MET-addicted’ cancers
Journal of Experimental & Clinical Cancer Research (2022)
-
Personalized therapeutic strategies in HER2-driven gastric cancer
Gastric Cancer (2021)
-
Linking drug target and pathway activation for effective therapy using multi-task learning
Scientific Reports (2018)