Abstract
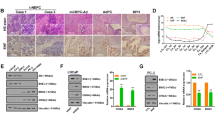
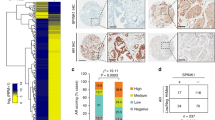
Prostatic neuroendocrine cells (NE) are an integral part of prostate cancer (PCa) and are associated with PCa progression. As the current androgen deprivation therapy with anti-androgens may promote the neuroendocrine PCa (NEPCa) development, and few therapies can effectively suppress NEPCa, understanding the impact of NEPCa on PCa progression may help us to develop better therapies to battle PCa. Here, we found NEPCa cells could increase the docetaxel resistance of their neighboring PCa cells. Mechanism dissection revealed that through secretion of PTHrP, NEPCa cells could alter the p38/MAPK/Hsp27 signals in their neighboring PCa cells that resulted in increased androgen receptor (AR) activity via promoting AR nuclear translocation. The consequences of increased AR function might then increase docetaxel resistance via increasing p21 expression. In vivo xenograft mice experiments also confirmed that NEPCa could increase the docetaxel resistance of neighboring PCa, and targeting this newly identified PTHrP/p38/Hsp27/AR/p21 signaling pathway with either p38 inhibitor (SB203580) or shPTHrP may result in improving/restoring the docetaxel sensitivity to better suppress PCa.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A . Global cancer statistics, 2012. CA Cancer J Clin 2015; 65: 87–108.
Karantanos T, Corn PG, Thompson TC . Prostate cancer progression after androgen deprivation therapy: mechanisms of castrate resistance and novel therapeutic approaches. Oncogene 2013; 32: 5501–5511.
Pienta KJ, Smith DC . Advances in prostate cancer chemotherapy: a new era begins. CA Cancer J Clin 2005; 55: 300–318 quiz 23-5.
Berthold DR, Pond GR, Soban F, de Wit R, Eisenberger M, Tannock IF . Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer: updated survival in the TAX 327 study. J Clin Oncol 2008; 26: 242–245.
Tannock IF, de Wit R, Berry WR, Horti J, Pluzanska A, Chi KN et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med 2004; 351: 1502–1512.
Terry S, Beltran H . The many faces of neuroendocrine differentiation in prostate cancer progression. Front Oncol 2014; 4: 60.
Yuan TC, Veeramani S, Lin FF, Kondrikou D, Zelivianski S, Igawa T et al. Androgen deprivation induces human prostate epithelial neuroendocrine differentiation of androgen-sensitive LNCaP cells. Endocr Relat Cancer 2006; 13: 151–167.
Abrahamsson PA . Neuroendocrine cells in tumour growth of the prostate. Endocr Relat Cancer 1999; 6: 503–519.
Levine L, Lucci JA 3rd, Pazdrak B, Cheng JZ, Guo YS, Townsend CM Jr. et al. Bombesin stimulates nuclear factor kappa B activation and expression of proangiogenic factors in prostate cancer cells. Cancer Res 2003; 63: 3495–3502.
DaSilva J, Gioeli D, Weber MJ, Parsons SJ . The neuroendocrine-derived peptide parathyroid hormone-related protein promotes prostate cancer cell growth by stabilizing the androgen receptor. Cancer Res 2009; 69: 7402–7411.
Conn EM, Botkjaer KA, Kupriyanova TA, Andreasen PA, Deryugina EI, Quigley JP . Comparative analysis of metastasis variants derived from human prostate carcinoma cells: roles in intravasation of VEGF-mediated angiogenesis and uPA-mediated invasion. Am J Pathol 2009; 175: 1638–1652.
DaSilva JO, Amorino GP, Casarez EV, Pemberton B, Parsons SJ . Neuroendocrine-derived peptides promote prostate cancer cell survival through activation of IGF-1 R signaling. Prostate 2013; 73: 801–812.
Jin RJ, Lho Y, Connelly L, Wang Y, Yu X, Saint Jean L et al. The nuclear factor-kappaB pathway controls the progression of prostate cancer to androgen-independent growth. Cancer Res 2008; 68: 6762–6769.
Wang ZQ, Stingl L, Morrison C, Jantsch M, Los M, Schulze-Osthoff K et al. PARP is important for genomic stability but dispensable in apoptosis. Genes Dev 1997; 11: 2347–2358.
Zhang XQ, Kondrikov D, Yuan TC, Lin FF, Hansen J, Lin MF . Receptor protein tyrosine phosphatase alpha signaling is involved in androgen depletion-induced neuroendocrine differentiation of androgen-sensitive LNCaP human prostate cancer cells. Oncogene 2003; 22: 6704–6716.
Chung LY, Tang SJ, Sun GH, Chou TY, Yeh TS, Yu SL et al. Galectin-1 promotes lung cancer progression and chemoresistance by upregulating p38 MAPK, ERK, and cyclooxygenase-2. Clin Cancer Res 2012; 18: 4037–4047.
Small GW, Shi YY, Higgins LS, Orlowski RZ . Mitogen-activated protein kinase phosphatase-1 is a mediator of breast cancer chemoresistance. Cancer Res 2007; 67: 4459–4466.
Marengo B, De Ciucis CG, Ricciarelli R, Furfaro AL, Colla R, Canepa E et al. p38MAPK inhibition: a new combined approach to reduce neuroblastoma resistance under etoposide treatment. Cell Death Dis 2013; 4: e589.
Bruey JM, Ducasse C, Bonniaud P, Ravagnan L, Susin SA, Diaz-Latoud C et al. Hsp27 negatively regulates cell death by interacting with cytochrome c. Nat Cell Biol 2000; 2: 645–652.
O'Shaughnessy RF, Welti JC, Cooke JC, Avilion AA, Monks B, Birnbaum MJ et al. AKT-dependent HspB1 (Hsp27) activity in epidermal differentiation. J Biol Chem 2007; 282: 17297–17305.
Hsu HS, Lin JH, Huang WC, Hsu TW, Su K, Chiou SH et al. Chemoresistance of lung cancer stemlike cells depends on activation of Hsp27. Cancer 2011; 117: 1516–1528.
Hadchity E, Aloy MT, Paulin C, Armandy E, Watkin E, Rousson R et al. Heat shock protein 27 as a new therapeutic target for radiation sensitization of head and neck squamous cell carcinoma. Mol Ther 2009; 17: 1387–1394.
Xu L, Chen S, Bergan RC . MAPKAPK2 and HSP27 are downstream effectors of p38 MAP kinase-mediated matrix metalloproteinase type 2 activation and cell invasion in human prostate cancer. Oncogene 2006; 25: 2987–2998.
Migliaccio A, Castoria G, Di Domenico M, de Falco A, Bilancio A, Lombardi M et al. Steroid-induced androgen receptor-oestradiol receptor beta-Src complex triggers prostate cancer cell proliferation. EMBO J 2000; 19: 5406–5417.
Lin J, Adam RM, Santiestevan E, Freeman MR . The phosphatidylinositol 3'-kinase pathway is a dominant growth factor-activated cell survival pathway in LNCaP human prostate carcinoma cells. Cancer Res 1999; 59: 2891–2897.
Berchem GJ, Bosseler M, Sugars LY, Voeller HJ, Zeitlin S, Gelmann EP . Androgens induce resistance to bcl-2-mediated apoptosis in LNCaP prostate cancer cells. Cancer Res 1995; 55: 735–738.
Zoubeidi A, Zardan A, Beraldi E, Fazli L, Sowery R, Rennie P et al. Cooperative interactions between androgen receptor (AR) and heat-shock protein 27 facilitate AR transcriptional activity. Cancer Res 2007; 67: 10455–10465.
Lu S, Jenster G, Epner DE . Androgen induction of cyclin-dependent kinase inhibitor p21 gene: role of androgen receptor and transcription factor Sp1 complex. Mol Endocrinol 2000; 14: 753–760.
Geng H, Rademacher BL, Pittsenbarger J, Huang CY, Harvey CT, Lafortune MC et al. ID1 enhances docetaxel cytotoxicity in prostate cancer cells through inhibition of p21. Cancer Res 2010; 70: 3239–3248.
Bhardwaj A, Srivastava SK, Singh S, Arora S, Tyagi N, Andrews J et al. CXCL12/CXCR4 signaling counteracts docetaxel-induced microtubule stabilization via p21-activated kinase 4-dependent activation of LIM domain kinase 1. Oncotarget 2014; 5: 11490–11500.
Shah GV, Thomas S, Muralidharan A, Liu Y, Hermonat PL, Williams J et al. Calcitonin promotes in vivo metastasis of prostate cancer cells by altering cell signaling, adhesion, and inflammatory pathways. Endocr Relat Cancer 2008; 15: 953–964.
Dizeyi N, Hedlund P, Bjartell A, Tinzl M, Austild-Tasken K, Abrahamsson PA . Serotonin activates MAP kinase and PI3K/Akt signaling pathways in prostate cancer cell lines. Urol Oncol 2011; 29: 436–445.
Marin-Aguilera M, Codony-Servat J, Reig O, Lozano JJ, Fernandez PL, Pereira MV et al. Epithelial-to-mesenchymal transition mediates docetaxel resistance and high risk of relapse in prostate cancer. Mol Cancer Ther 2014; 13: 1270–1284.
Yuan J, Rozengurt E . PKD, PKD2, and p38 MAPK mediate Hsp27 serine-82 phosphorylation induced by neurotensin in pancreatic cancer PANC-1 cells. J Cell Biochem 2008; 103: 648–662.
Heron-Milhavet L, LeRoith D . Insulin-like growth factor I induces MDM2-dependent degradation of p53 via the p38 MAPK pathway in response to DNA damage. J Biol Chem 2002; 277: 15600–15606.
Kim J, Song G, Gao H, Farmer JL, Satterfield MC, Burghardt RC et al. Insulin-like growth factor II activates phosphatidylinositol 3-kinase-protooncogenic protein kinase 1 and mitogen-activated protein kinase cell Signaling pathways, and stimulates migration of ovine trophectoderm cells. Endocrinology 2008; 149: 3085–3094.
Guo YS, Hellmich MR, Wen XD, Townsend CM Jr. . Activator protein-1 transcription factor mediates bombesin-stimulated cyclooxygenase-2 expression in intestinal epithelial cells. J Biol Chem 2001; 276: 22941–22947.
Park SI, Lee C, Sadler WD, Koh AJ, Jones J, Seo JW et al. Parathyroid hormone-related protein drives a CD11b+Gr1+ cell-mediated positive feedback loop to support prostate cancer growth. Cancer Res 2013; 73: 6574–6583.
Jin RJ, Wang Y, Masumori N, Ishii K, Tsukamoto T, Shappell SB et al. NE-10 neuroendocrine cancer promotes the LNCaP xenograft growth in castrated mice. Cancer Res 2004; 64: 5489–5495.
Domingo-Domenech J, Vidal SJ, Rodriguez-Bravo V, Castillo-Martin M, Quinn SA, Rodriguez-Barrueco R et al. Suppression of acquired docetaxel resistance in prostate cancer through depletion of notch- and hedgehog-dependent tumor-initiating cells. Cancer Cell 2012; 22: 373–388.
Thadani-Mulero M, Portella L, Sun S, Sung M, Matov A, Vessella RL et al. Androgen receptor splice variants determine taxane sensitivity in prostate cancer. Cancer Res 2014; 74: 2270–2282.
Zhu Y, Liu C, Nadiminty N, Lou W, Tummala R, Evans CP et al. Inhibition of ABCB1 expression overcomes acquired docetaxel resistance in prostate cancer. Mol Cancer Ther 2013; 12: 1829–1836.
Hirano D, Okada Y, Minei S, Takimoto Y, Nemoto N . Neuroendocrine differentiation in hormone refractory prostate cancer following androgen deprivation therapy. Eur Urol 2004; 45: 586–592 discussion 92.
Qi J, Nakayama K, Cardiff RD, Borowsky AD, Kaul K, Williams R et al. Siah2-dependent concerted activity of HIF and FoxA2 regulates formation of neuroendocrine phenotype and neuroendocrine prostate tumors. Cancer Cell 2010; 18: 23–38.
Dang Q, Li L, Xie H, He D, Chen J, Song W et al. Anti-androgen enzalutamide enhances prostate cancer neuroendocrine (NE) differentiation via altering the infiltrated mast cells —> androgen receptor (AR) —> miRNA32 signals. Mol Oncol 2015; 9: 1241–1251.
Xiao D, Qu X, Weber HC . GRP receptor-mediated immediate early gene expression and transcription factor Elk-1 activation in prostate cancer cells. Regul Pept 2002; 109: 141–148.
Dizeyi N, Bjartell A, Nilsson E, Hansson J, Gadaleanu V, Cross N et al. Expression of serotonin receptors and role of serotonin in human prostate cancer tissue and cell lines. Prostate 2004; 59: 328–336.
Salido M, Vilches J, Roomans GM . Changes in elemental concentrations in LNCaP cells are associated with a protective effect of neuropeptides on etoposide-induced apoptosis. Cell Biol Int 2004; 28: 397–402.
Vilches J, Salido M, Fernandez-Segura E, Roomans GM . Neuropeptides, apoptosis and ion changes in prostate cancer. Methods of study and recent developments. Histol Histopathol 2004; 19: 951–961.
Harper ME, Glynne-Jones E, Goddard L, Thurston VJ, Griffiths K . Vascular endothelial growth factor (VEGF) expression in prostatic tumours and its relationship to neuroendocrine cells. Br J Cancer 1996; 74: 910–916.
Bulavin DV, Higashimoto Y, Popoff IJ, Gaarde WA, Basrur V, Potapova O et al. Initiation of a G2/M checkpoint after ultraviolet radiation requires p38 kinase. Nature 2001; 411: 102–107.
Xia Z, Dickens M, Raingeaud J, Davis RJ, Greenberg ME . Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science 1995; 270: 1326–1331.
Corcelle E, Djerbi N, Mari M, Nebout M, Fiorini C, Fenichel P et al. Control of the autophagy maturation step by the MAPK ERK and p38: lessons from environmental carcinogens. Autophagy 2007; 3: 57–59.
Razandi M, Pedram A, Levin ER . Heat shock protein 27 is required for sex steroid receptor trafficking to and functioning at the plasma membrane. Mol Cell Biol 2010; 30: 3249–3261.
Jia Y, Ransom RF, Shibanuma M, Liu C, Welsh MJ, Smoyer WE . Identification and characterization of hic-5/ARA55 as an hsp27 binding protein. J Biol Chem 2001; 276: 39911–39918.
Lamoureux F, Thomas C, Yin MJ, Fazli L, Zoubeidi A, Gleave ME . Suppression of heat shock protein 27 using OGX-427 induces endoplasmic reticulum stress and potentiates heat shock protein 90 inhibitors to delay castrate-resistant prostate cancer. Eur Urol 2014; 66: 145–155.
Zhao Y, Shen S, Guo J, Chen H, Greenblatt DY, Kleeff J et al. Mitogen-activated protein kinases and chemoresistance in pancreatic cancer cells. J Surg Res 2006; 136: 325–335.
Jazirehi AR, Vega MI, Chatterjee D, Goodglick L, Bonavida B . Inhibition of the Raf-MEK1/2-ERK1/2 signaling pathway, Bcl-xL down-regulation, and chemosensitization of non-Hodgkin's lymphoma B cells by Rituximab. Cancer Res 2004; 64: 7117–7126.
Zelivianski S, Spellman M, Kellerman M, Kakitelashvilli V, Zhou XW, Lugo E et al. ERK inhibitor PD98059 enhances docetaxel-induced apoptosis of androgen-independent human prostate cancer cells. Int J Cancer 2003; 107: 478–485.
Thadani-Mulero M, Nanus DM, Giannakakou P . Androgen receptor on the move: boarding the microtubule expressway to the nucleus. Cancer Res 2012; 72: 4611–4615.
Fizazi K, Faivre L, Lesaunier F, Delva R, Gravis G, Rolland F et al. Androgen deprivation therapy plus docetaxel and estramustine versus androgen deprivation therapy alone for high-risk localised prostate cancer (GETUG 12): a phase 3 randomised controlled trial. Lancet Oncol 2015; 16: 787–794.
Cerasuolo M, Paris D, Iannotti FA, Melck D, Verde R, Mazzarella E et al. Neuroendocrine transdifferentiation in human prostate cancer cells: an integrated approach. Cancer Res 2015; 75: 2975–2986.
Fan W, Yanase T, Morinaga H, Okabe T, Nomura M, Daitoku H et al. Insulin-like growth factor 1/insulin signaling activates androgen signaling through direct interactions of Foxo1 with androgen receptor. J Biol Chem 2007; 282: 7329–7338.
Leotoing L, Manin M, Monte D, Baron S, Communal Y, Lours C et al. Crosstalk between androgen receptor and epidermal growth factor receptor-signalling pathways: a molecular switch for epithelial cell differentiation. J Mol Endocrinol 2007; 39: 151–162.
Hobisch A, Eder IE, Putz T, Horninger W, Bartsch G, Klocker H et al. Interleukin-6 regulates prostate-specific protein expression in prostate carcinoma cells by activation of the androgen receptor. Cancer Res 1998; 58: 4640–4645.
Ongkeko WM, Burton D, Kiang A, Abhold E, Kuo SZ, Rahimy E et al. Parathyroid hormone related-protein promotes epithelial-to-mesenchymal transition in prostate cancer. PloS One 2014; 9: e85803.
Liao J, Li X, Koh AJ, Berry JE, Thudi N, Rosol TJ et al. Tumor expressed PTHrP facilitates prostate cancer-induced osteoblastic lesions. Int J Cancer 2008; 123: 2267–2278.
Niu Y, Altuwaijri S, Lai KP, Wu CT, Ricke WA, Messing EM et al. Androgen receptor is a tumor suppressor and proliferator in prostate cancer. Proc Natl Acad Sci USA 2008; 105: 12182–12187.
Hu S, Li L, Yeh S, Cui Y, Li X, Chang HC et al. Infiltrating T cells promote prostate cancer metastasis via modulation of FGF11—>miRNA-541—>androgen receptor (AR)—>MMP9 signaling. Mol Oncol 2015; 9: 44–57.
Acknowledgements
This work was supported by NIH grants (CA155477 and CA156700), George Whipple Professorship Endowment and Taiwan Department of Health Clinical Trial, Research Center of Excellence (DOH99-TD-B-111-004 to China Medical University, Taichung, Taiwan) and China 973 Program (2012CB518305). We thank Karen Wolf for help in preparing the manuscript.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Rights and permissions
About this article
Cite this article
Cui, Y., Sun, Y., Hu, S. et al. Neuroendocrine prostate cancer (NEPCa) increased the neighboring PCa chemoresistance via altering the PTHrP/p38/Hsp27/androgen receptor (AR)/p21 signals. Oncogene 35, 6065–6076 (2016). https://doi.org/10.1038/onc.2016.135
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2016.135
This article is cited by
-
LncRNA-p21 alters the antiandrogen enzalutamide-induced prostate cancer neuroendocrine differentiation via modulating the EZH2/STAT3 signaling
Nature Communications (2019)
-
M2 macrophage-mediated interleukin-4 signalling induces myofibroblast phenotype during the progression of benign prostatic hyperplasia
Cell Death & Disease (2018)