Abstract
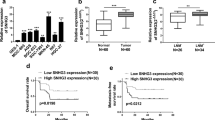
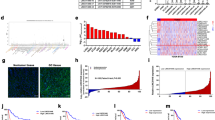
Recently, intriguing new roles for some small nucleolar RNA host genes (SNHGs) in cancer have emerged. In the present study, a panel of SNHGs was profiled to detect aberrantly expressed SNHGs in gastric cancer (GC). The expression of SNHG5 was significantly downregulated in GC and was significantly associated with the formation of a tumor embolus and with the tumor, node and metastasis stage. SNHG5 was a long non-coding RNA, which was a class of non-coding RNA transcripts longer than 200 nucleotides. SNHG5 suppressed GC cell proliferation and metastasis in vitro and in vivo. Furthermore, SNHG5 exerted its function through interacting with MTA2, preventing the translocation of MTA2 from the cytoplasm into the nucleus. SNHG5 overexpression led to significant increases in the acetylation levels of histone H3 and p53, indicating that SNHG5 might affect acetylation by trapping MTA2 in the cytosol, thereby interfering with the formation of the nucleosome remodeling and histone deacetylation complex. This study is the first to demonstrate that SNHG5 is a critical and powerful regulator that is involved in GC progression through trapping MTA2 in the cytosol. These results imply that SNHG5 may be a novel therapeutic target for the treatment of GC.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Nagini S . Carcinoma of the stomach: a review of epidemiology, pathogenesis, molecular genetics and chemoprevention. World J Gastrointest Oncol 2012; 4: 156–169.
Liao J, Yu L, Mei Y, Guarnera M, Shen J, Li R et al. Small nucleolar RNA signatures as biomarkers for non-small-cell lung cancer. Mol Cancer 2010; 9: 198.
Martens-Uzunova ES, Jalava SE, Dits NF, van Leenders GJ, Møller S, Trapman J et al. Diagnostic and prognostic signatures from the small non-coding RNA transcriptome in prostate cancer. Oncogene 2012; 31: 978–991.
Gee HE, Buffa FM, Camps C, Ramachandran A, Leek R, Taylor M et al. The small-nucleolar RNAs commonly used for microRNA normalisation correlate with tumour pathology and prognosis. Br J Cancer 2011; 104: 1168–1177.
Okugawa Y, Toiyama Y, Toden S, Mitoma H, Nagasaka T, Tanaka K et al. Clinical significance of SNORA42 as an oncogene and a prognostic biomarker in colorectal cancer. Gut 2015, e-pub ahead of print 15 October 2015 doi:10.1136/gutjnl-2015-309359.
Askarian-Amiri ME, Crawford J, French JD, Smart CE, Smith MA, Clark MB et al. SNORD-host RNA Zfas1 is a regulator of mammary development and a potential marker for breast cancer. RNA 2011; 17: 878–891.
Williams GT, Hughes JP, Stoneman V, Anderson CL, McCarthy NJ, Mourtada-Maarabouni M et al. Isolation of genes controlling apoptosis through their effects on cell survival. Gene Ther Mol Biol 2006; 10B: 255–261.
Kino T, Hurt DE, Ichijo T, Nader N, Chrousos GP, Noncoding RNA . Gas5 is a growth arrest-and starvation-associated repressor of the glucocorticoid receptor. Sci Signal 2010; 3: ra8.
Mourtada-Maarabouni M, Pickard MR, Hedge VL, Farzaneh F, Williams GT . GAS5, a non-protein-coding RNA, controls apoptosis and is downregulated in breast cancer. Oncogene 2009; 28: 195–208.
Hudson WH, Pickard MR, de Vera IM, Kuiper EG, Mourtada-Maarabouni M, Conn GL et al. Conserved sequence-specific lincRNA-steroid receptor interactions drive transcriptional repression and direct cell fate. Nat Commun 2014; 5: 5395.
Yang F, Bi J, Xue X, Zheng L, Zhi K, Hua et al. Up-regulated long non-coding RNA H19 contributes to proliferation of gastric cancer cells. FEBS J 2012; 279: 3159–3165.
Okugawa Y, Toiyama Y, Hur K, Toden S, Saigusa S, Tanaka K et al. Metastasis-associated long non-coding RNA drives gastric cancer development and promotes peritoneal metastasis. Carcinogenesis 2014; 35: 2731–2739.
Mizrahi I, Mazeh H, Grinbaum R, Beglaibter N, Wilschanski M, Pavlov V et al. Colon cancer associated transcript-1 (CCAT1) expression in adenocarcinoma of the stomach. J Cancer 2015; 6: 105–110.
Cao WJ, Wu HL, He BS, Zhang YS, Zhang ZY . Analysis of long non-coding RNA expression profiles in gastric cancer. World J Gastroenterol 2013; 19: 3658–3664.
Sun M, Jin FY, Xia R, Kong R, Li JH, Xu TP et al. Decreased expression of long noncoding RNA GAS5 indicates a poor prognosis and promotes cell proliferation in gastric cancer. BMC Cancer 2014; 14: 319.
Tanaka R, Satoh H, Moriyama M, Satoh K, Morishita Y, Yoshida S et al. Intronic U50 small-nuclear-RNA (snoRNA) host gene of no protein-coding potential is mapped at the chromosome breakpoint t(3;6)(q27;q15) of human B-cell lymphoma. Genes Cells 2000; 5: 277–287.
Dong XY, Rodriguez C, Guo P, Sun X, Talbot JT, Zhou W et al. SnoRNA U50 is a candidate tumor-suppressor gene at 6q14.3 with a mutation associated with clinically significant prostate cancer. Hum Mol Genet 2008; 17: 1031–1042.
Bowen NJ, Fujita N, Kajita M, Wade PA . Mi-2/NuRD: multiple complexes for many purposes. Biochim Biophys Acta 2004; 1677: 52–57.
Zhou C, Ji J, Cai Q, Shi M, Chen X, Yu Y et al. MTA2 promotes gastric cancer cells invasion and is transcriptional regulated by Sp1. Mol Cancer 2012; 12: 102.
Covington KR, Brusco L, Barone I, Tsimelzon A, Selever J, Corona-Rodriguez A et al. Metastasis tumor-associated protein 2 enhances metastatic behavior and is associated with poor outcomes in estrogen receptor-negative breast cancer. Breast Cancer Res Treat 2013; 141: 375–384.
Lee H, Ryu SH, Hong SS, Seo DD, Min HJ, Jang MK et al. Overexpression of metastasis-associated protein 2 is associated with hepatocellular carcinoma size and differentiation. J Gastroenterol Hepatol 2009; 24: 1445–1450.
Luo J, Su F, Chen D, Shiloh A, Gu W . Deacetylation of p53 modulates its effect on cell growth and apoptosis. Nature 2000; 408: 377–381.
Wu M, Wang L, Li Q, Li J, Qin J, Wong J . The MTA family proteins as novel histone H3 binding proteins. Cell Bio Sci 2013; 3: 1.
Fu J, Qin L, He T, Qin J, Hong J, Wong J et al. The TWIST/Mi2/NuRD protein complex and its essential role in cancer metastasis. Cell Res 2011; 21: 275–289.
Li J, Zhang L, Gao Z, Kang H, Rong G, Zhang X et al. Dual inhibition of EGFR at protein and activity level via combinatorial blocking of PI4KIIα as anti-tumor strategy. Protein Cell 2014; 5: 457–468.
Scarpino S, Duranti E, Giglio S, Di Napoli A, Galafate D, Del Bufalo D et al. Papillary carcinoma of the thyroid: high expression of COX-2 and low expression of KAI-1/CD82 are associated with increased tumor invasiveness. Thyroid 2013; 23: 1127–1137.
Dong XY, Guo P, Boyd J, Sun X, Li Q, Zhou W et al. Implication of snoRNA U50 in human breast cancer. J Genet Genomics 2009; 36: 447–454.
Chaudhry MA . Expression pattern of small nucleolar RNA host genes and long non-coding RNA in X-rays-treated lymphoblastoid cells. Int J Mol Sci 2013; 14: 9099–9110.
Siprashvili Z, Webster DE, Johnston D, Shenoy RM, Ungewickell AJ, Bhaduri A et al. The noncoding RNAs SNORD50A and SNORD50B bind K-Ras and are recurrently deleted in human cancer. Nat Genet 2015; 48: 53–58.
Acknowledgements
This work was partially supported by the State Key Basic Research Program of China (Grant No. 2013CB530805, to J Shi and D Zheng), the Natural Science Foundation of China (Grant No. 81372200, 81572755, to J Shi), the PUMC Youth Fund and the Fundamental Research Funds for the Central Universities (Grant No. 3332013055 and 3332014007, to J Shi).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Zhao, L., Guo, H., Zhou, B. et al. Long non-coding RNA SNHG5 suppresses gastric cancer progression by trapping MTA2 in the cytosol. Oncogene 35, 5770–5780 (2016). https://doi.org/10.1038/onc.2016.110
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2016.110
This article is cited by
-
An increase in SNHG5 expression is associated with poor cancer prognosis, according to a meta-analysis
European Journal of Medical Research (2024)
-
RNF144A-AS1, a TGF-β1- and hypoxia-inducible gene that promotes tumor metastasis and proliferation via targeting the miR-30c-2-3p/LOX axis in gastric cancer
Cell & Bioscience (2021)
-
Long non-coding RNAs in Epstein–Barr virus-related cancer
Cancer Cell International (2021)
-
si-SNHG5-FOXF2 inhibits TGF-β1-induced fibrosis in human primary endometrial stromal cells by the Wnt/β-catenin signalling pathway
Stem Cell Research & Therapy (2020)
-
LncRNA SNHG5 promotes nasopharyngeal carcinoma progression by regulating miR-1179/HMGB3 axis
BMC Cancer (2020)