Abstract
In mice, the deletion of either Mdm2 or Mdm4 results in a p53-dependent embryonic lethality. We used zinc-finger nucleases to construct mutations in the mdm2 and mdm4 genes of zebrafish. Although the loss of mdm2 results in a p53-dependent early embryonic lethality, mdm4 mutant fish are viable and grow to adulthood. We also found that an in-frame five-amino acid deletion in mdm2 creates a novel hypomorphic allele. The lethal phenotype observed in the mdm2 mutant fish could be partially rescued by injecting mRNA encoding functional Mdm2, and this required the E3 ligase activity of the protein. Complete rescue was obtained by crossing the mdm2 mutant fish onto a p53M214K mutant background. Although p53 mutant fish on a wild-type mdm2 background were shown to accumulate high levels of p53 protein specifically in tumor tissues, we detected extensive staining of p53 in many normal tissues of the mdm2–p53M214K double-mutant fish. Our results are suggestive of the hypothesis that p53 protein accumulates during tumor formation as a result of tumor-specific inactivation of the Mdm2 pathway.
Similar content being viewed by others
Introduction
p53, the guardian of the genome, has been shown to be activated in response to a myriad of signals, including, but not limited to, DNA damage, oncogenic activation, hypoxic, metabolic and ribosomal biogenesis stresses.1, 2 In more than 50% of human cancers, the p53 gene is either mutated, or its activity disrupted by the deregulation of other genes in the p53 pathway.3 Mdm2 is the primary ubiquitin E3 ligase that inhibits p53 activity by binding to the transactivation domain of p53,4, 5 as well as undermining p53 protein stability by targeting it for ubiquitination and subsequent proteosomal degradation.6, 7 Its structural homolog, Mdm4, is also able to bind and inhibit p53-mediated transcription.8 Mdm4 possesses a C-terminal RING domain, but it lacks intrinsic ubiquitin E3 ligase activity. Instead, it was reported to stabilize Mdm2 via a RING–RING interaction9 and to regulate the ubiquitination of p53 and Mdm2.10 In genetic mouse models, Mdm2 and Mdm4 were shown to be essential and nonredundant in regulating p53 activity in vivo, and their heterodimerization was seen to be particularly important in embryogenesis.11, 12, 13
With the exception of the two invertebrate model organisms, Drosophila melanogaster and Caenorhabditis elegans, both p53 and Mdm2 are conserved through 1.5 billion years of evolution, with the earliest ortholog found in the primitive organism Trichoplax adhaerens.14, 15 In zebrafish, similar to mammals, p53 is activated in response to DNA-damaging signals,16 and the use of morpholinos in transient knockdown assays demonstrated a lethality in embryos caused by a lack of Mdm2, which could be rescued by a concomitant knockdown of p53.17 This observation is similar to what was shown in Mdm2-null mice,18, 19 suggesting that the epistasis between p53 and Mdm2 is also conserved in zebrafish.
In this study, we exploit the advances in gene-editing technology to create targeted mutations in the mdm2 and mdm4 loci in zebrafish to generate genetic models that confirm the epistasis of p53 and Mdm2, and also to enable us to investigate the involvement of Mdm4 in the regulation of zebrafish p53. Consistent with results from the mouse knockout studies, the functional deletion of mdm2 in zebrafish led to embryonic lethality caused by p53 accumulation, which leads to extensive apoptosis, and this lethality can be completely rescued by mutating both alleles of p53. In contrast, deleting most of the zebrafish mdm4 gene does not appear to affect growth and development, indicating some evolutionary differences between the p53 pathway in fish and mammals.
A striking similarity between the regulation of p53 in zebrafish and mouse was observed in the stability of mutant p53 protein. In normal mouse or zebrafish tissues, mutant p53 protein levels are undetectable but are stabilized and increased dramatically in response to DNA damaging or other stress signals due to the temporary and reversible inactivation of Mdm2.20, 21 In contrast, in most tumors that arise in animals expressing mutant p53, p53 protein is stabilized even in the absence of any external damage signal.20, 21, 22 This is quite different from p53 mutant Mdm2-null mice,21 where mutant p53 now accumulates in some, but not all, normal tissues. This accumulation of mutant p53 was also seen in the mdm2–p53 double-mutant zebrafish we generated, but not in the mdm4–p53 double mutants. The inactivation of mdm2 in these fish led to the dramatic accumulation of mutant p53 in many tissues, lending strong support to the hypothesis that the inactivation of Mdm2 function in p53 degradation is a universal feature of vertebrate cancers.
Results
Targeted disruption of the zebrafish mdm2 and mdm4 genes
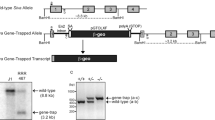
Custom-designed zinc-finger nucleases (ZFNs) were used to target the genomic loci of mdm2 and mdm4 in zebrafish (Figure 1a). Chimeric F0 embryos were grown to adulthood and outcrossed with wild-type fish to identify lineages that carried germline mutations from the founders. Genomic DNA was extracted from the scales of F1 fish to PCR amplify and sequence the genomic region containing the zinc-finger nuclease-binding and -cut site. A total of four mdm2 mutant alleles and three mdm4 mutant alleles were identified from the screening (Figures 1b and c). To confirm that these mutations were also present after transcription, total RNA was extracted from F2 embryos and sequenced after RT–PCR. mdm2Δ5 is a 5-base pair deletion in the coding sequence of mdm2; mdm2+2 and mdm2+4 are insertions of 2 and 4 base pairs, respectively; and mdm2Δ15represents an in-frame deletion of 15 base pairs. Likewise, mdm4Δ5 and mdm4Δ8 represent 5- and 8-base pair deletions in the mdm4 coding sequence, whereas mdm4sub5(+7) denotes a 5-base pair substitution and a 7-base pair insertion.
Targeted disruption of zebrafish mdm2 and mdm4 using ZFNs. (a) A schematic representation of the genomic loci of zebrafish mdm2 on chromosome 4 and mdm4 on chromosome 11. The red arrow indicates the zinc-finger-binding and -cut site. Various known protein domains are indicated in different colors: cyan, Mdm2/SWIB (p53-binding domain); yellow, RanBP2-type zinc-finger; fuschia, RING finger. (b) Alignments of partial coding sequences isolated from heterozygous F1 zebrafish carrying mdm2 mutant alleles. (c) Alignments of partial coding sequences isolated from heterozygous F1 zebrafish carrying mdm4 mutant alleles.
The F1 fish carrying a single mutant allele for either mdm2 or mdm4 are visually indistinguishable from their wild-type siblings and are viable and fertile. Three of the mutant alleles in the mdm2 gene (mdm2Δ5, mdm2+2 and mdm2+4) are predicted to yield truncated protein products owing to an introduction of a premature stop codon near the zinc-finger-binding site (Supplementary Figure 1a). As there are no noticeable phenotypic differences between the homozygous mutants of these three alleles, they will be referred to as mdm2 functional knockouts (mdm2−/−) from hereon. The mdm2Δ15allele is expected to yield a Mdm2 protein that has a loss of five amino acids (residues 144–148) that will not affect its C-terminal RING domain (Supplementary Figure 1a). On the other hand, all three mdm4 mutant alleles are predicted to generate truncated protein products that are likely to be nonfunctional (Supplementary Figure 1b), and will be referred to as mdm4 functional knockouts (mdm4−/−). In addition, a molecular model of the interaction between zebrafish mdm4 protein and a p53 peptide shows that a truncated mdm4 protein lacks the residues required for binding to p53 (Supplementary Figure 2).
mdm2, but not mdm4, is an essential gene in zebrafish embryos
Langheinrich et al.17 performed a transient knockdown of mdm2 in zebrafish embryos using antisense morpholinos, showing that reducing the endogenous levels of Mdm2 causes an increase in the p53-dependent apoptotic activity and indicating that Mdm2 is required in zebrafish embryonic development to keep the activity of p53 in check. However, it has been reported that morpholinos may elicit off-target effects caused by a nonspecific activation of the p53 pathway,23 making it complicated to distinguish between a specific biological function of Mdm2 and an off-target effect of the morpholino. To address this, a genetic mutant would be a more ideal tool for studying the in vivo functions of zebrafish Mdm2.
Similar to Mdm2-null mice, the genetic loss of functional Mdm2 in zebrafish results in lethality.18, 19 mdm2−/−, but not the mdm2Δ15/Δ15 fish, are embryonic lethal and do not survive beyond 96 h post fertilization (hpf; Table 1). mdm2−/− embryos were obtained in Mendelian ratio from an incross of mdm2+/− parents. These embryos were indistinguishable from their wild-type and heterozygote siblings (wild type/mdm2+/−) at earlier stages of embryogenesis (up to 5–6 somite stages), but begin to display a darkening of the tissue in and around the head at the 10-somite stage (12 hpf; Figure 2a). By 24 hpf, the mdm2−/− embryos displayed a lethal phenotype—the eyes were malformed, the trunk of the embryo failed to extend sufficiently and no circulation could be detected as a result of failure in heart development (data not shown). The inability of the mdm2−/−embryos to develop normally was likely to be caused by excessive cell death, and this was confirmed by positive staining for acridine orange and an increase in Caspase 3/7 activity in the cell lysate obtained from the embryos (Figures 2b and c).
Embryonic development and analysis of cell death in wild-type and mdm2−/− embryos. Embryos were obtained from an incross of wild-type or mdm2+/− zebrafish. (a) Brightfield images of wild-type and mdm2−/−embryos at matching developmental stages show the lethal phenotype due to the homozygous loss of mdm2. (b) The 24-hpf wild-type and mdm2−/− embryos were stained live with acridine orange and imaged under ultraviloet light. Increased green fluorescence in mdm2−/− embryos compared with wild-type embryos suggests the presence of more apoptotic cells. (c) Whole-cell lysate was prepared from a number of wild-type/mdm2+/− and mdm2−/− embryos and used in a Caspase 3/7 assay. Cells from mdm2−/− embryos have higher Caspase 3/7 activity compared with wild-type/mdm2+/− siblings.
Mdm4-null mice are embryonic lethal at a later developmental stage than that in Mdm2-null mice. Mdm4 was shown to regulate the p53 activity in spatially and temporally distinct way from Mdm2, whereas the Mdm2-null mice showed induction of apoptosis, the Mdm4-null mice displayed inhibited proliferation due to cell cycle arrest.24, 25 However, in this study, none of the zebrafish mdm4 homozygous mutants were embryonic lethal (Table 1 and Supplementary Figure 3), suggesting an evolutionary divergence in Mdm4 function in fish and mammals.
The ubiquitin E3 ligase activity of Mdm2 is vital for embryonic survival in zebrafish
To confirm that the embryonic lethal phenotype is due to the loss of functional Mdm2 in zebrafish, up to 50 pg of FLAG-mdm2 mRNA was synthesized and microinjected into all the embryos obtained from an incross of mdm2+/− fish. Upon observation at 30 hpf, less than a quarter of the injected embryos exhibited the severe phenotype of mdm2−/− fish. A unit of 25 pg of FLAG-mdm2 mRNA was sufficient to cause a significant improvement in the development of the eye and the extension of the trunk of the mdm2−/− embryos (Figures 3a–d and Supplementary Table 2). The exogenous expression of mdm2 thus partially rescued the lethality in mdm2−/− embryos. Mutating a single-zinc-coordinating cysteine residue in the murine Mdm2 RING domain has been shown to disrupt the ubiquitin E3 ligase activity of Mdm2 without impairing its binding to p53.26, 27 The zebrafish equivalent of this mutation, C448A, was introduced into the rescue mRNA. This ligase-dead mdm2-encoding mRNA failed to rescue the phenotype of the mdm2−/− embryos (Figures 3e and f), demonstrating the importance of the function of mdm2 as an ubiquitin E3 ligase in zebrafish embryogenesis. In addition, this rescue assay also establishes a system that allows the analysis of Mdm2 activity or p53 inhibition in a whole animal system.
Rescue of mdm2−/− embryos with microinjection of FLAG-mdm2 mRNA. Single-cell embryos collected from an incross of mdm2+/− fish were injected with 25 or 50 pg of mRNA and imaged at ~30 hpf. (a) Wild type/mdm2+/− sibling embryo; (b) uninjected mdm2−/−embryo; (c) mdm2−/− embryo injected with 25 pg FLAG-mdm2 mRNA; (d) mdm2−/− embryo injected with 50 pg FLAG-mdm2 mRNA; (e) mdm2−/− embryo injected with 25 pg FLAG-mdm2C448A mRNA; and (f) mdm2−/− embryo injected with 50 pg FLAG-mdm2C448A mRNA.
Mdm2 is an important regulator of p53 levels and activity in zebrafish embryos
The complete loss of Mdm2 results in an increase in p53 protein and activity, causing cell death and embryonic lethality in mice.18, 19 To show that the three mutant alleles of zebrafish mdm2−/− are comparable to the Mdm2-null genotype in mice, the levels of p53 protein and activity were analyzed in the mdm2−/− embryos. Embryos obtained from an incross mdm2+/− fish were collected and the mdm2−/− embryos could be visually distinguished from their wild-type/mdm2+/− siblings. Whole mount immunohistochemistry for p53 showed that mdm2−/− embryos accumulate high levels of p53 protein in vivo compared with their wild-type/mdm2+/− siblings (Figure 4a).
mdm2−/− embryos accumulate high levels of p53 protein and have increased p53 target gene transcription. (a) Whole mount immunohistochemistry was performed on embryos from an incross of mdm2+/− fish. The embryos were fixed in methanol:acetone (1:1) at the six-somite stage and stained with p53-5.1 hybridoma supernatant (green) and DAPI counterstain (blue). The stained embryos were deyolked, dorsally mounted in glycerol and imaged at the trunk at a × 40 magnification on a confocal microscope. (b) Western blot analysis on lysates of embryos from different incrosses—wild type, mdm2Δ15/15, mdm2+/−, p53M214K/M214K and mdm2−/−; p53M214K/M214K. A unit of 20 Gy gamma irradiation was performed on 24 hpf embryos and the total lysate from all embryos was collected at 28 hpf. A unit of 30 μg of lysate was loaded in each lane of the SDS–PAGE gel. (c) Quantitative real-time PCR analysis of p53 target genes in wild-type and mutant mdm2 embryos. RNA was extracted from 50 embryos at 24 hpf in TRIzol, and purified before utilizing 1 μg of total RNA in a reverse transcription reaction. A volume of 0.5 μl of each cDNA was used in a quantitative real-time PCR reaction set up in triplicate in a 384-well plate. The gene expression data was analyzed using actin and EF1a as internal controls. *P<0.05 for Student’s t-test.
A western blot analysis was subsequently performed on 28 hpf embryos for a semiquantitative comparison of p53 protein levels. Similar to wild-type embryos, the wild-type/mdm2+/− siblings only accumulated p53 protein when they were subjected to 20 Gy gamma irradiation at 24 hpf (Figure 4b, lanes 1, 2, 5 and 6). On the other hand, the mdm2−/−embryos showed high levels of p53 protein even without the genotoxic treatment (Figure 4b, lane 7). The level of p53 protein in the mdm2Δ15/Δ15 embryos was also undetectable without any genotoxic treatment, but upon gamma irradiation, they appear to accumulate more p53 protein than wild-type fish (Figure 4b, lanes 3 and 4). This suggests that the short five-amino acid deletion in this mdm2 mutant may confer a hypomorphic phenotype when cells are challenged with a stress signal. More experiments need to be conducted to further confirm this hypothesis.
Quantitative real-time PCR was also performed to confirm the correlation between increased p53 protein levels and its transactivation activity. As expected, an increase in the transcript levels of p53-responsive genes, such as Δ113p53, mdm2, p21, PUMA and Bax, was observed for the mdm2−/− embryos but not in their wild-type/mdm2+/− siblings (Figure 4c). It should be noted that the primers for amplifying the Mdm2 transcript lie upstream of the zinc-finger nuclease-binding site and is thus able to detect the truncated mRNA in mdm2−/− embryos. In these experiments, p53M214K/M214K embryos were used as a negative control as p53M214K encodes a transcriptionally inactive protein.28 Also consistent with mdm2Δ15 being a hypomorphic allele, higher levels of expression of some p53 reporter genes compared with wild-type fish were detected following gamma irradiation (Figure 4c). This was particularly noticeable for mdm2, p21, PUMA and Bax.
Lethality of the mdm2−/− genotype is p53-dependent and can be rescued by mutations in both copies of p53 gene
To show that the lethality of the homozygous loss of functional Mdm2 in zebrafish is p53-dependent, mdm2+/− fish were crossed with p53M214K/M214K fish. Double heterozygotes mdm2+/−; p53+/M214K were identified by genotyping and were subsequently incrossed. Although a single mutant copy of p53 rescued the embryonic lethal phenotype of the mdm2−/− fish (Supplementary Figure 3), none of these mdm2−/−; p53+/M214K fish survived to adulthood, suggesting the haploinsufficiency of p53 to counteract the homozygous loss of mdm2. On the other hand, mutating both copies of the p53 gene completely rescues the mdm2−/− fish: mdm2−/−; p53M214K/M214K fish are viable and fertile (Supplementary Figure 3). This suggests that the M214K mutation thoroughly inactivates p53 and the rescue is consistent with previous work done in the Mdm2–p53 mutant mice.21
Mdm2 (but not Mdm4) regulates levels of mutant p53 in embryos and adult fish
Earlier work in our laboratory described how while mutant p53 is expressed at low and undetectable levels in normal zebrafish tissues, a genotoxic stress such as gamma irradiation could induce the accumulation and persistence of mutant p53 protein.20 Terzian et al.21 crossed Mdm2-null mice with p53 mutant mice and demonstrated that the homozygous loss of Mdm2 resulted in the stabilization of mutant p53 protein in several tissues. To find out if the regulation of mutant p53 by Mdm2 is conserved in zebrafish, western blot and histological analyses were performed on embryos and paraffin sections of 5-month-old adult zebrafish.
Our results showed that the loss of functional Mdm2 results in an increase in mutant p53 protein even from the embryonic stage (Figure 4b last lane, Figure 5 and Supplementary Figure 6) up until adulthood (Figure 6). In the mdm2−/−; p53M214K/M214K adult zebrafish sections, mutant p53 staining was observed in several tissues such as the skin, gut, kidney and liver, whereas in the p53M214K/M214K fish, p53 staining was undetectable (Figure 6, top and bottom rows). Interestingly, the intense staining for p53 detected in zebrafish kidneys is consistent with observations made by Zhang et al.29 in the kidneys of Mdm2 conditional knockout mice.
mdm2−/−; p53M214K/M214K embryos accumulate mutant p53 protein. Whole mount immunohistochemistry was performed on mdm2−/−; p53M214K/M214K and wild-type embryos at 30 hpf. The embryos were fixed in methanol:acetone (1:1) and stained with p53-5.1 hybridoma supernatant (green) and DAPI counterstain (blue). The stained embryos were deyolked, dorsally mounted in glycerol and imaged at the midbrain at × 40 magnification using a confocal microscope. (a, b) Magnified insets showing different mutant p53 localization in different cells in the mdm2−/−; p53M214K/M214K embryo.
mdm2−/−; p53M214K/M214K adult zebrafish accumulate mutant p53 protein in most tissues. Five-month-old mdm2−/−; p53M214K/M214K, mdm4−/−; p53M214K/M214K and p53M214K/M214K adult zebrafish were sacrificed, fixed and embedded in paraffin for histological analysis with the p53-5.1 hybridoma supernatant. The sections were counterstained with hematoxylin and imaged under brightfield at × 40 magnification. E, epidermis; CE, columnar epithelium; CSM, circular smooth muscle; T, renal tubule; H, hepatocytes; S, sinusoid
In the different tissues of the mdm2−/−; p53M214K/M214K fish, the distribution of mutant p53 staining shows variability (Figure 6, top row). For example, in the epidermal layer of the skin and columnar epithelium of the gut, p53 staining appears uniform and intense throughout. In the gut, however, staining is exclusive to the columnar epithelium: no p53 staining is detected for the circular smooth muscle cells. In addition, the cells lining the renal tubules show variation in their intensities for p53 staining. The staining detected in hepatocytes of the liver also appears uniform, albeit less intense than in the epithelial cells. The hematopoietic cells detected in the sinusoids of the liver also lack p53 staining. To rule out that the distribution of p53 staining might be dependent on the proliferation status of a cell, serial sections of the gut were stained for the proliferative marker, proliferating cell nuclear antigen (Supplementary Figure 5). The results showed that p53 accumulation did not only coincide with the regions where cells were proliferating. This is consistent with earlier findings in mouse that Mdm2 is vital in maintaining low p53 levels in both dividing and terminally differentiated cells.30
In addition, as Mdm4 is expected to regulate p53 activity and not stability,30 our results also show that Mdm4 does not have a role in maintaining the levels of wild-type or mutant p53 in zebrafish—p53 staining could not be detected in mdm4−/−or mdm4−/−; p53M214K/M214K fish (Supplementary Figure 4 and Figure 6, middle row). These observations suggest that while Mdm2 (and not Mdm4) is likely to have a major role in keeping the levels of mutant p53 low in most zebrafish tissues, its activity could vary depending on the cell type.
Zebrafish tumors accumulate mutant p53 like mdm2−/−; p53M214K/M214K zebrafish
It has been shown that about 28% of p53M214K/M214K adult fish develop tumors from 16.5 months of age.28 p53M214K/M214K adult fish with tumors were sacrificed, and their tumors removed for histological analyses. These tumors, which are commonly found in the eye and the trunk, stained strongly for p53 (Figure 7). Similar to mdm2−/−; p53M214K/M214K fish, there is heterogeneity in the staining: most but not all of the nuclei stain positive for p53. As these fish carry wild-type mdm2 alleles, the results are suggestive that in zebrafish tumors, the accumulation of mutant p53 is caused by an inactivation of wild-type Mdm2 activity by a yet-to-be determined mechanism.
Accumulation of mutant p53 protein in tumors of p53M214K/M214K fish. Adult p53M214K/M214K zebrafish that were observed to have developed tumors in the eye and trunk were sacrificed, fixed and embedded in paraffin for histological analysis with the p53-5.1 hybridoma supernatant. The sections were counterstained with hematoxylin and imaged under brightfield. (a) Normal eye from a p53M214K/M214K fish ( × 10 magnification). (b) Tumor in the eye of a p53M214K/M214K fish ( × 10 magnification). (c) Tumor in the eye of a p53M214K/M214K fish ( × 40 magnification). (d) Tumor in the trunk of a p53M214K/M214K fish ( × 40 magnification). P, pigmentation of eye lens.
Discussion
ZFNs have been used efficiently to generate genetic knockouts of several zebrafish genes.31, 32 In this study, to generate genetic models of zebrafish that lack functional Mdm2 or Mdm4 protein, we designed two pairs of ZFNs to target the respective loci in zebrafish. Although the roles of both Mdm2 and Mdm4 in regulating p53 have been studied extensively in mouse models,18, 19, 25, 30, 33 it was of interest to find out if these observations were also conserved in zebrafish, particularly for Mdm4, because the mechanisms by which it regulates p53 remains contentious.
In mice, one model proposed that Mdm2 and Mdm4 function in nonoverlapping pathways. This was demonstrated in the embryonic lethality of Mdm4-null mice due to unchecked p53 transcriptional activity that leads to cell cycle arrest,25, 34, 35 and that Mdm4 regulates p53 transcriptional activity independent of Mdm2 in vivo.30 The second model proposed that Mdm4 functions with mutual dependence on Mdm2. One paper highlighted the importance of Mdm4 forming heterodimers with Mdm2 in embryonic development but not in adult tissues,13 whereas another study showed that the overexpression of Mdm2 could compensate for the loss of Mdm4,33 suggesting that Mdm4 is dispensable if sufficient Mdm2 is present to suppress p53 protein levels.
We were hoping that the Mdm4 mutant zebrafish would give an unambiguous perspective on the in vivo role of Mdm4. In contrast to the Mdm4-null mice, we found that all the mutant mdm4 alleles did not affect the embryonic development of zebrafish. As the ancestor of zebrafish is known to have undergone large scale gene duplication,36 we wondered if there was another copy of mdm4 in zebrafish that could have compensated the mutations we made. A thorough search through the databases was performed and it was confirmed that there was only one copy of the mdm4 gene in zebrafish (personal communication with Dr Venkatesh Byrappa, IMCB, Singapore). The mdm4 mutant alleles were predicted to yield truncated proteins of only about 60 amino acids, and they lack the C-terminal RING domain that was shown, in mouse, to be essential for its function.12, 13 In addition, a homology model of the N-terminus of Mdm4 and a p53 peptide further demonstrated that a truncated 64-amino acid Mdm4 would fail to bind to p53 (Supplementary Figure 2). These results indicate that if zebrafish Mdm4 had a role in regulating p53 in a similar way as was demonstrated in mice, we would expect the homozygous mdm4 mutants to be embryonic lethal. Therefore, a possible explanation for this is that the role of Mdm4 in the p53 pathway evolved after the divergence of teleost and mammals. A supporting evidence for this hypothesis is the absence of a known zebrafish ortholog of the Ptprv gene. Ptprv is a p53-inducible gene that encodes a protein tyrosine phosphatase receptor essential for mediating p53-dependent cell cycle arrest, and was found to be highly upregulated in Mdm4 mutant mice.37 Thus, Mdm4 appears to be either a nonessential and/or redundant gene in zebrafish, at least with respect to p53 regulation.
We do not rule out the possibility that Mdm4 might have other p53-independent roles and will use the mdm4–p53 double-mutant zebrafish as a tool to further explore other biological functions of Mdm4. This is of particular importance to understand the possible side effects of drugs designed to inhibit Mdm4.
Homozygous mutants of three zebrafish mdm2 mutant alleles (mdm2Δ5, mdm2+2 and mdm2+4) displayed an embryonic lethal phenotype akin to the Mdm2-null or mutant mice.18, 19, 27 Even though there have been reports of 14 other ubiquitin E3 and E4 proteins that have orthologs in zebrafish (online database search) and can target p53 and regulate its stability (reviewed in Pant and Lozano),38 Mdm2 has a dominant role in the regulation of p53 levels in zebrafish, at least in early development. We demonstrated that the embryonic lethal phenotype of the mdm2 mutants could be partially rescued by the exogenous expression of functional Mdm2 (Figure 3). This rescue experiment is potentially useful for the functional analyses of Mdm2 from various species or in testing inhibitors of p53 activity, especially as the sequence and structure of Mdm2 and p53 are highly conserved across zebrafish, mouse and humans.14
In this paper, we have also isolated a non-lethal mutant allele of Mdm2, mdm2Δ15. This mutant lacks only five amino acids in the Mdm2 protein and develops normally to adulthood. Interestingly, the loss of these five residues confers a hypomorphic phenotype when the fish are exposed to an external damage signal—these mutants appear to elicit a stronger p53 response compared with wild-type fish (Figure 4). We have not tested to see whether this mutant exhibits radiosensitivity similar to that of an Mdm2-hypomorphic mouse.39 More experiments have to be performed to elucidate the biological relevance of these five residues and why they might be important in moderating the accumulation of p53 in response to DNA damage.
There is much clinical significance behind the efforts to understand the mechanisms that regulate the stability of p53 mutant proteins because mutations in p53 are associated with poor prognosis in several human tumors and are likely to affect cellular responses to chemotherapy.40 Heterozygous mouse models carrying a single allele of mutant p53 exhibited p53 accumulation in tumors but not in normal cells,41, 42 suggesting that this stabilization of mutant p53 is attributed solely not only to the mutation in p53 but also to a secondary event that is present in a tumor environment. This tumor-specific accumulation of mutant p53 in p53 mutant zebrafish was also presented in this paper (Figure 7).
In our study, we generated mdm2–p53 double-mutant zebrafish that demonstrated two things: (1) the epistasis between Mdm2 and p53 is highly conserved from fish to mammals: mutating both alleles of p53 rescues the lethal phenotype of mdm2 mutant fish; and (2) similar to mice,21 Mdm2 has a key role in regulating the turnover of mutant p53 protein, and this function is inactivated in most tumor cells. Although it is tempting to claim that Mdm2 is the core switch that controls the stability of mutant p53, it may not be the only regulator. This is evident in the heterogeneity of p53 staining in the mdm2–p53 double mutants and also in zebrafish tumors. It would be interesting to find out why some of these cells apparently retain the normal instability of mutant p53 and why Mdm2 is dispensable in these cells. Other mechanisms that limit the accumulation of p53 may include regulation of its promoter and, as implied by studies of ribosomal protein mutants,2 mechanisms that function at the level of the p53 mRNA. In mice, a major route by which p53 can be stabilized is through the action of the p19ARF protein, but as zebrafish lack an ARF ortholog, the tumor-specific accumulation of mutant p53 in zebrafish must be mediated by other signaling pathways. Discerning the exact nature of these pathways is now open to a genetic approach utilizing the powerful tools available in the zebrafish system.
Materials and Methods
Zebrafish husbandry
Adult zebrafish (Danio rerio) were maintained with a controlled light cycle of 14 h light/10 h dark at 28 °C. Embryos were cultured in egg water (0.3% w/v sea-salt solution) at 28.5 °C until the desired stage. All experiments were conducted in accordance to the ethical guidelines and approved by the Institutional Animal Care and Use Committee, Biomedical Research Council.
ZFNs
Sequences of the ZFNs are shown in Supplementary Table 1 (Sigma Aldrich, St Louis, MO, USA).
Genomic DNA extraction and genotyping
Scales from adult zebrafish were digested in scale lysis buffer (10 mm Tris-HCl, pH 8.0, 50 mm KCl, 0.5%(v/v) Tween 20, 0.3%(v/v) Nonidet P-40 and 0.22 mg/ml proteinase-K) at 55 °C. Proteinase-K was inactivated at 95 °C for 5 min. A volume of 3 μl of the reaction was used for PCR. Primer pairs used to amplify mdm2 and mdm4 alleles are listed in Supplementary Table 3. The PCR reactions were incubated with Exonuclease I and Shrimp Antarctic Phosphatase (New England Biolabs, Inc., Ipswich, MA, USA) at 37 °C for 30 min and inactivated at 80 °C for 8 min before sequencing.
Acridine orange staining
Embryos were incubated with a 0.1% acridine orange solution in egg water and imaged with the Leica M165FC fluorescence stereomicroscope (Leica, Solms, Germany).
Caspase 3/7 assay
The embryos were lysed by sonication in lysis buffer (10 mm Tris-HCl, pH 7.5, 10 mm NaCl, 0.5% Nonidet P-40, protease inhibitor cocktail (Roche, Basel, Switzerland)). Caspase-Glo 3/7 reagent (Promega, Madison, WI, USA) was added in a 1:1 ratio with the cleared lysate and incubated in the dark for 2 h. Luminescence was measured using the EnVision plate reader (PerkinElmer, Waltham, MA, USA).
Plasmids and mRNA synthesis
The mdm2 coding sequence (Ensembl Transcript ID: ENSDART00000077854, Zv9) was cloned downstream of FLAG in a PCS2+ vector using primers in Supplementary Table 3. The C448A mutation was introduced by site-directed mutagenesis using primers in Supplementary Table 3. FLAG–mdm2 and FLAG–mdm2(C448A) mRNA were synthesized with the mMESSAGE SP6 Kit (Ambion, Carlsbad, CA, USA) according to the manufacturers’ instructions.
Immunohistochemistry
Whole mount immunohistochemistry of p53 in zebrafish embryos was carried out as previously described.20 AlexaFluor 488 Rabbit Anti-Mouse IgG (Molecular Probes, Life Technologies, Carlsbad, CA, USA) was used for the secondary antibody and the embryos were imaged using Zeiss LSM510 confocal microscope (Carl Zeiss AG, Oberkochen, Germany).
Adult zebrafish were fixed in Dietrich’s fixative before embedding in paraffin, at the Advanced Molecular Pathology Laboratory, IMCB, A*STAR, Singapore. The paraffin blocks were processed and previously described by Guo et al.20 ZFp53-5.1 hybridoma supernatant16 was used neat for the primary antibody and the peroxidase-labeled Goat Anti-Mouse Ig (Dako, Carpinteria, CA, USA) was diluted 1:2 and used as the secondary antibody. Antibody staining was performed as described by Lee et al.16 The slides were imaged on the Zeiss AxioImager Z1 microscope and analyzed with AxioVision 4.8 software (Carl Zeiss AG).
Protein extraction and western blot
The embryos were deyolked and sonicated in cell lysis buffer. Thirty micrograms of denatured protein lysate was loaded on each lane of the SDS–PAGE gel. ZFp53-9.1 hybridoma supernatant was diluted 1:2 for the primary antibody. The secondary antibody was horseradish peroxidase-conjugated Rabbit Anti-Mouse diluted 1:1000 (Dako). Horseradish peroxidase-conjugated Donkey Anti-Actin Antibody (Santa Cruz Biotechnology, Dallas, TX, USA) was used at a 1:2000 dilution as a loading control. The membrane was incubated with chemiluminescent substrate (Nacalai USA, Inc., San Diego, CA, USA) before exposure and analysis Image Studio 3.1.4 (Li-COR Inc., Lincoln, NE, USA).
Real-time quantitative RT-PCR
Embryos were homogenized in TRIzol (Invitrogen, Waltham, CA, USA) according to the manufacturer’s instructions. A unit of 1 μg of total RNA was reverse transcribed to cDNA using iScript Reverse Transcription Supermix (Bio-Rad Laboratories, Hercules, CA, USA). Real-time quantitative PCR reaction using SSoAdvanced Universal SYBR Green Supermix (Bio-Rad Laboratories) was set up according to the manufacturer’s protocol. The primers are listed in Supplementary Table 4.
References
Vousden KH, Lu X . Live or let die: the cell's response to p53. Nat Rev Cancer 2002; 2: 594–604.
MacInnes AW, Amsterdam A, Whittaker CA, Hopkins N, Lees JA . Loss of p53 synthesis in zebrafish tumors with ribosomal protein gene mutations. Proc Natl Acad Sci USA 2008; 105: 10408–10413.
Hollstein M, Sidransky D, Vogelstein B, Harris CC . p53 mutations in human cancers. Science 1991; 253: 49–53.
Momand J, Zambetti GP, Olson DC, George D, Levine AJ . The mdm-2 oncogene product forms a complex with the p53 protein and inhibits p53-mediated transactivation. Cell 1992; 69: 1237–1245.
Oliner JD, Pietenpol JA, Thiagalingam S, Gyuris J, Kinzler KW, Vogelstein B . Oncoprotein MDM2 conceals the activation domain of tumour suppressor p53. Nature 1993; 362: 857–860.
Yang Y, Li CC, Weissman AM . Regulating the p53 system through ubiquitination. Oncogene 2004; 23: 2096–2106.
Fang S, Jensen JP, Ludwig RL, Vousden KH, Weissman AM . Mdm2 is a RING finger-dependent ubiquitin protein ligase for itself and p53. J Biol Chem 2000; 275: 8945–8951.
Shvarts A, Steegenga WT, Riteco N, van Laar T, Dekker P, Bazuine M et al. MDMX: a novel p53-binding protein with some functional properties of MDM2. EMBO J 1996; 15: 5349–5357.
Sharp DA, Kratowicz SA, Sank MJ, George DL . Stabilization of the MDM2 oncoprotein by interaction with the structurally related MDMX protein. J Biol Chem 1999; 274: 38189–38196.
Linares LK, Hengstermann A, Ciechanover A, Muller S, Scheffner M . HdmX stimulates Hdm2-mediated ubiquitination and degradation of p53. Proc Natl Acad Sci USA 2003; 100: 12009–12014.
Marine JC, Francoz S, Maetens M, Wahl G, Toledo F, Lozano G . Keeping p53 in check: essential and synergistic functions of Mdm2 and Mdm4. Cell Death Differ 2006; 13: 927–934.
Huang L, Yan Z, Liao X, Li Y, Yang J, Wang ZG et al. The p53 inhibitors MDM2/MDMX complex is required for control of p53 activity in vivo. Proc Natl Acad Sci USA 2011; 108: 12001–12006.
Pant V, Xiong S, Iwakuma T, Quintas-Cardama A, Lozano G . Heterodimerization of Mdm2 and Mdm4 is critical for regulating p53 activity during embryogenesis but dispensable for p53 and Mdm2 stability. Proc Natl Acad Sci USA 2011; 108: 11995–12000.
Lane DP, Cheok CF, Brown C, Madhumalar A, Ghadessy FJ, Verma C . Mdm2 and p53 are highly conserved from placozoans to man. Cell Cycle 2010; 9: 540–547.
Lane DP, Verma C . Mdm2 in evolution. Genes Cancer 2012; 3: 320–324.
Lee KC, Goh WL, Xu M, Kua N, Lunny D, Wong JS et al. Detection of the p53 response in zebrafish embryos using new monoclonal antibodies. Oncogene 2008; 27: 629–640.
Langheinrich U, Hennen E, Stott G, Vacun G . Zebrafish as a model organism for the identification and characterization of drugs and genes affecting p53 signaling. Curr Biol 2002; 12: 2023–2028.
Jones SN, Roe AE, Donehower LA, Bradley A . Rescue of embryonic lethality in Mdm2-deficient mice by absence of p53. Nature 1995; 378: 206–208.
Montes de Oca Luna R, Wagner DS, Lozano G . Rescue of early embryonic lethality in mdm2-deficient mice by deletion of p53. Nature 1995; 378: 203–206.
Guo L, Liew HP, Camus S, Goh AM, Chee LL, Lunny DP et al. Ionizing radiation induces a dramatic persistence of p53 protein accumulation and DNA damage signaling in mutant p53 zebrafish. Oncogene 2013; 32: 4009–4016.
Terzian T, Suh YA, Iwakuma T, Post SM, Neumann M, Lang GA et al. The inherent instability of mutant p53 is alleviated by Mdm2 or p16INK4a loss. Genes Dev 2008; 22: 1337–1344.
Hall PA, Lane DP . p53 in tumour pathology: can we trust immunohistochemistry?—Revisited!. J Pathol 1994; 172: 1–4.
Robu ME, Larson JD, Nasevicius A, Beiraghi S, Brenner C, Farber SA et al. p53 activation by knockdown technologies. PLoS Genet 2007; 3: e78.
Chavez-Reyes A, Parant JM, Amelse LL, de Oca Luna RM, Korsmeyer SJ, Lozano G . Switching mechanisms of cell death in mdm2- and mdm4-null mice by deletion of p53 downstream targets. Cancer Res 2003; 63: 8664–8669.
Parant J, Chavez-Reyes A, Little NA, Yan W, Reinke V, Jochemsen AG et al. Rescue of embryonic lethality in Mdm4-null mice by loss of Trp53 suggests a nonoverlapping pathway with MDM2 to regulate p53. Nat Genet 2001; 29: 92–95.
Geyer RK, Yu ZK, Maki CG . The MDM2 ING-finger domain is required to promote p53 nuclear export. Nat Cell Biol 2000; 2: 569–573.
Itahana K, Mao H, Jin A, Itahana Y, Clegg HV, Lindstrom MS et al. Targeted inactivation of Mdm2 RING finger E3 ubiquitin ligase activity in the mouse reveals mechanistic insights into p53 regulation. Cancer Cell 2007; 12: 355–366.
Berghmans S, Murphey RD, Wienholds E, Neuberg D, Kutok JL, Fletcher CD et al. tp53 mutant zebrafish develop malignant peripheral nerve sheath tumors. Proc Natl Acad Sci USA 2005; 102: 407–412.
Zhang Y, Xiong S, Li Q, Hu S, Tashakori M, Van Pelt C et al. Tissue-specific and age-dependent effects of global Mdm2 loss. J Pathol 2014; 233: 380–391.
Francoz S, Froment P, Bogaerts S, De Clercq S, Maetens M, Doumont G et al. Mdm4 and Mdm2 cooperate to inhibit p53 activity in proliferating and quiescent cells in vivo. Proc Natl Acad Sci USA 2006; 103: 3232–3237.
Meng X, Noyes MB, Zhu LJ, Lawson ND, Wolfe SA . Targeted gene inactivation in zebrafish using engineered zinc-finger nucleases. Nat Biotechnol 2008; 26: 695–701.
Doyon Y, McCammon JM, Miller JC, Faraji F, Ngo C, Katibah GE et al. Heritable targeted gene disruption in zebrafish using designed zinc-finger nucleases. Nat Biotechnol 2008; 26: 702–708.
Steinman HA, Hoover KM, Keeler ML, Sands AT, Jones SN . Rescue of Mdm4-deficient mice by Mdm2 reveals functional overlap of Mdm2 and Mdm4 in development. Oncogene 2005; 24: 7935–7940.
Finch RA, Donoviel DB, Potter D, Shi M, Fan A, Freed DD et al. mdmx is a negative regulator of p53 activity in vivo. Cancer Res 2002; 62: 3221–3225.
Migliorini D, Lazzerini Denchi E, Danovi D, Jochemsen A, Capillo M, Gobbi A et al. Mdm4 (Mdmx) regulates p53-induced growth arrest and neuronal cell death during early embryonic mouse development. Mol Cell Biol 2002; 22: 5527–5538.
Taylor JS, Braasch I, Frickey T, Meyer A, Van de Peer Y . Genome duplication, a trait shared by 22000 species of ray-finned fish. Genome Res 2003; 13: 382–390.
Doumont G, Martoriati A, Beekman C, Bogaerts S, Mee PJ, Bureau F et al. G1 checkpoint failure and increased tumor susceptibility in mice lacking the novel p53 target Ptprv. EMBO J 2005; 24: 3093–3103.
Pant V, Lozano G . Limiting the power of p53 through the ubiquitin proteasome pathway. Genes Dev 2014; 28: 1739–1751.
Mendrysa SM, McElwee MK, Michalowski J, O'Leary KA, Young KM, Perry ME . mdm2 Is critical for inhibition of p53 during lymphopoiesis and the response to ionizing irradiation. Mol Cell Biol 2003; 23: 462–472.
Peller S . Clinical implications of p53: effect on prognosis, tumor progression and chemotherapy response. Semin Cancer Biol 1998; 8: 379–387.
Lang GA, Iwakuma T, Suh YA, Liu G, Rao VA, Parant JM et al. Gain of function of a p53 hot spot mutation in a mouse model of Li-Fraumeni syndrome. Cell 2004; 119: 861–872.
Olive KP, Tuveson DA, Ruhe ZC, Yin B, Willis NA, Bronson RT et al. Mutant p53 gain of function in two mouse models of Li-Fraumeni syndrome. Cell 2004; 119: 847–860.
Acknowledgements
This work was supported by the Biomedical Research Council of Singapore. We thank Dr Verma Chandra, Bioinformatics Institute, Singapore, for the homology modeling of Mdm4 and p53; Dr Venkatesh Byrappa, Institute of Molecular and Cell Biology, Singapore, for scouring the databases for zebrafish Mdm4 homologs; Dr Julin Wong for growing the hybridomas for the p53-5.1 and p53-9.1 antibodies; and our students who helped with genotyping of the zebrafish. The zebrafish p53M214K mutant line was provided by Professor A. Thomas Look (Dana Farber Cancer Institute, Boston, MA, USA). Adult zebrafish were processed and paraffin embedded at the Advanced Molecular Pathology Laboratory, IMCB, A*STAR, Singapore. All the adult zebrafish were maintained by the Zebrafish Facility at the Institute of Molecular and Cell Biology.
Author Contributions
DPL conceived the study; LG designed and injected the ZFNs, HPL studied the embryonic development of the mutants and performed the whole mount immunohistochemistry of zebrafish embryos; and JSC carried out all the other experiments. The data was analyzed by JSC, HPL and DPL. The manuscript was written by JSC and DPL, with input from HPL.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
About this article
Cite this article
Chua, J., Liew, H., Guo, L. et al. Tumor-specific signaling to p53 is mimicked by Mdm2 inactivation in zebrafish: insights from mdm2 and mdm4 mutant zebrafish. Oncogene 34, 5933–5941 (2015). https://doi.org/10.1038/onc.2015.57
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2015.57
This article is cited by
-
Puma, noxa, p53, and p63 differentially mediate stress pathway induced apoptosis
Cell Death & Disease (2021)
-
A subset of SMN complex members have a specific role in tissue regeneration via ERBB pathway-mediated proliferation
npj Regenerative Medicine (2020)
-
A novel molecular rotor facilitates detection of p53-DNA interactions using the Fluorescent Intercalator Displacement Assay
Scientific Reports (2018)