Abstract
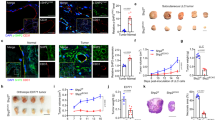
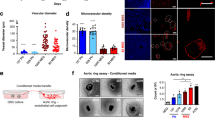
We identify a limited subpopulation of epidermal cancer stem cells (ECS cells), in squamous cell carcinoma, that form rapidly growing, invasive and highly vascularized tumors, as compared with non-stem cancer cells. These ECS cells grow as non-attached spheroids, and display enhanced migration and invasion. We show that ECS cell-produced vascular endothelial growth factor (VEGF)-A is required for the maintenance of this phenotype, as knockdown of VEGF-A gene expression or treatment with VEGF-A-inactivating antibody reduces these responses. In addition, treatment with bevacizumab reduces tumor vascularity and growth. Surprisingly, the classical mechanism of VEGF-A action via interaction with VEGF receptors does not mediate these events, as these cells lack VEGFR1 and VEGFR2. Instead, VEGF-A acts via the neuropilin-1 (NRP-1) co-receptor. Knockdown of NRP-1 inhibits ECS cell spheroid formation, invasion and migration, and attenuates tumor formation. These studies suggest that VEGF-A acts via interaction with NRP-1 to trigger intracellular events leading to ECS cell survival and formation of aggressive, invasive and highly vascularized tumors.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
American Cancer Society Website. Cancer Facts and Figures. Available at: http://www.cancer.org/, 2010.
Bickers DR, Lim HW, Margolis D, Weinstock MA, Goodman C, Faulkner E et al. The burden of skin diseases: 2004 a joint project of the American Academy of Dermatology Association and the Society for Investigative Dermatology. J Am Acad Dermatol 2006; 55: 490–500.
Rogers HW, Weinstock MA, Harris AR, Hinckley MR, Feldman SR, Fleischer AB et al. Incidence estimate of nonmelanoma skin cancer in the United States, 2006. Arch Dermatol 2010; 146: 283–287.
Pleasance ED, Cheetham RK, Stephens PJ, McBride DJ, Humphray SJ, Greenman CD et al. A comprehensive catalogue of somatic mutations from a human cancer genome. Nature 2010; 463: 191–196.
Folkman J . The role of angiogenesis in tumor growth. Semin Cancer Biol 1992; 3: 65–71.
Johnson KE, Wilgus TA . Multiple roles for VEGF in non-melanoma skin cancer: angiogenesis and beyond. J Skin Cancer 2012; 2012: 483439.
Claesson-Welsh L, Welsh M . VEGFA and tumour angiogenesis. J Intern Med 2013; 273: 114–127.
Koch S, Tugues S, Li X, Gualandi L, Claesson-Welsh L . Signal transduction by vascular endothelial growth factor receptors. Biochem J 2011; 437: 169–183.
Mueller MM, Fusenig NE . Tumor-stroma interactions directing phenotype and progression of epithelial skin tumor cells. Differentiation 2002; 70: 486–497.
Hirakawa S, Kodama S, Kunstfeld R, Kajiya K, Brown LF, Detmar M . VEGF-A induces tumor and sentinel lymph node lymphangiogenesis and promotes lymphatic metastasis. J Exp Med 2005; 201: 1089–1099.
Larcher F, Murillas R, Bolontrade M, Conti CJ, Jorcano JL . VEGF/VPF overexpression in skin of transgenic mice induces angiogenesis, vascular hyperpermeability and accelerated tumor development. Oncogene 1998; 17: 303–311.
Beck B, Driessens G, Goossens S, Youssef KK, Kuchnio A, Caauwe A et al. A vascular niche and a VEGF-Nrp1 loop regulate the initiation and stemness of skin tumours. Nature 2011; 478: 399–403.
Lichtenberger BM, Tan PK, Niederleithner H, Ferrara N, Petzelbauer P, Sibilia M . Autocrine VEGF signaling synergizes with EGFR in tumor cells to promote epithelial cancer development. Cell 2010; 140: 268–279.
Wilgus TA, Matthies AM, Radek KA, Dovi JV, Burns AL, Shankar R et al. Novel function for vascular endothelial growth factor receptor-1 on epidermal keratinocytes. Am J Pathol 2005; 167: 1257–1266.
Zhu JW, Wu XJ, Lu ZF, Luo D, Cai SQ, Zheng M . Role of VEGF receptors in normal and psoriatic human keratinocytes: evidence from irradiation with different UV sources. PLoS One 2013; 8: e55463.
Zhu JW, Wu XJ, Luo D, Lu ZF, Cai SQ, Zheng M . Activation of VEGFR-2 signaling in response to moderate dose of ultraviolet B promotes survival of normal human keratinocytes. Int J Biochem Cell Biol 2012; 44: 246–256.
Man XY, Yang XH, Cai SQ, Yao YG, Zheng M . Immunolocalization and expression of vascular endothelial growth factor receptors (VEGFRs) and neuropilins (NRPs) on keratinocytes in human epidermis. Mol Med 2006; 12: 127–136.
Yang XH, Man XY, Cai SQ, Yao YG, Bu ZY, Zheng M . Expression of VEGFR-2 on HaCaT cells is regulated by VEGF and plays an active role in mediating VEGF induced effects. Biochem Biophys Res Commun 2006; 349: 31–38.
Bao S, Wu Q, Sathornsumetee S, Hao Y, Li Z, Hjelmeland AB et al. Stem cell-like glioma cells promote tumor angiogenesis through vascular endothelial growth factor. Cancer Res 2006; 66: 7843–7848.
Goel HL, Mercurio AM . VEGF targets the tumour cell. Nat Rev Cancer 2013; 13: 871–882.
Zhao Y, Bao Q, Renner A, Camaj P, Eichhorn M, Ischenko I et al. Cancer stem cells and angiogenesis. Int J Dev Biol 2011; 55: 477–482.
Casanova ML, Larcher F, Casanova B, Murillas R, Fernandez-Acenero MJ, Villanueva C et al. A critical role for ras-mediated, epidermal growth factor receptor-dependent angiogenesis in mouse skin carcinogenesis. Cancer Res 2002; 62: 3402–3407.
Linde N, Lederle W, Depner S, van RN, Gutschalk CM, Mueller MM . Vascular endothelial growth factor-induced skin carcinogenesis depends on recruitment and alternative activation of macrophages. J Pathol 2012; 227: 17–28.
Adhikary G, Grun D, Kerr C, Balasubramanian S, Rorke EA, Vemuri M et al. Identification of a population of epidermal squamous cell carcinoma cells with enhanced potential for tumor formation. PLoS One 2013; 8: e84324.
Eckert RL, Adhikary G, Balasubramanian S, Rorke EA, Vemuri MC, Boucher SE et al. Biochemistry of epidermal stem cells. Biochim Biophys Acta 2013; 1830: 2427–2434.
Harris AL . Hypoxia—a key regulatory factor in tumour growth. Nat Rev Cancer 2002; 2: 38–47.
Chun YS, Kim MS, Park JW . Oxygen-dependent and -independent regulation of HIF-1alpha. J Korean Med Sci 2002; 17: 581–588.
Semenza GL . Hypoxia-inducible factors: mediators of cancer progression and targets for cancer therapy. Trends Pharmacol Sci 2012; 33: 207–214.
Djordjevic S, Driscoll PC . Targeting VEGF signalling via the neuropilin co-receptor. Drug Discov Today 2013; 18: 447–455.
Borriello L, Montes M, Lepelletier Y, Leforban B, Liu WQ, Demange L et al. Structure-based discovery of a small non-peptidic Neuropilins antagonist exerting in vitro and in vivo anti-tumor activity on breast cancer model. Cancer Lett 2014; 349: 120–127.
Jarvis A, Allerston CK, Jia H, Herzog B, Garza-Garcia A, Winfield N et al. Small molecule inhibitors of the neuropilin-1 vascular endothelial growth factor A (VEGF-A) interaction. J Med Chem 2010; 53: 2215–2226.
Nissen JC, Selwood DL, Tsirka SE . Tuftsin signals through its receptor neuropilin-1 via the transforming growth factor beta pathway. J Neurochem 2013; 127: 394–402.
Boukamp P . Non-melanoma skin cancer: what drives tumor development and progression? Carcinogenesis 2005; 26: 1657–1667.
Pincelli C, Marconi A . Keratinocyte stem cells: friends and foes. J Cell Physiol 2010; 225: 310–315.
Al-Hajj M, Clarke MF . Self-renewal and solid tumor stem cells. Oncogene 2004; 23: 7274–7282.
Tosetti F, Ferrari N, De FS, Albini A . Angioprevention': angiogenesis is a common and key target for cancer chemopreventive agents. FASEB J 2002; 16: 2–14.
Adhikary G, Grun D, Balasubramanian S, Kerr C, Huang J, Eckert R . Survival of skin cancer stem cells requires the Ezh2 polycomb group protein. Carcinogenesis 2015; 36: 800–810.
Mamluk R, Gechtman Z, Kutcher ME, Gasiunas N, Gallagher J, Klagsbrun M . Neuropilin-1 binds vascular endothelial growth factor 165, placenta growth factor-2, and heparin via its b1b2 domain. J Biol Chem 2002; 277: 24818–24825.
Migdal M, Huppertz B, Tessler S, Comforti A, Shibuya M, Reich R et al. Neuropilin-1 is a placenta growth factor-2 receptor. J Biol Chem 1998; 273: 22272–22278.
Soker S, Takashima S, Miao HQ, Neufeld G, Klagsbrun M . Neuropilin-1 is expressed by endothelial and tumor cells as an isoform-specific receptor for vascular endothelial growth factor. Cell 1998; 92: 735–745.
Kitsukawa T, Shimono A, Kawakami A, Kondoh H, Fujisawa H . Overexpression of a membrane protein, neuropilin, in chimeric mice causes anomalies in the cardiovascular system, nervous system and limbs. Development 1995; 121: 4309–4318.
Takashima S, Kitakaze M, Asakura M, Asanuma H, Sanada S, Tashiro F et al. Targeting of both mouse neuropilin-1 and neuropilin-2 genes severely impairs developmental yolk sac and embryonic angiogenesis. Proc Natl Acad Sci USA 2002; 99: 3657–3662.
Akagi M, Kawaguchi M, Liu W, McCarty MF, Takeda A, Fan F et al. Induction of neuropilin-1 and vascular endothelial growth factor by epidermal growth factor in human gastric cancer cells. Br J Cancer 2003; 88: 796–802.
Bachelder RE, Crago A, Chung J, Wendt MA, Shaw LM, Robinson G et al. Vascular endothelial growth factor is an autocrine survival factor for neuropilin-expressing breast carcinoma cells. Cancer Res 2001; 61: 5736–5740.
Parikh AA, Fan F, Liu WB, Ahmad SA, Stoeltzing O, Reinmuth N et al. Neuropilin-1 in human colon cancer: expression, regulation, and role in induction of angiogenesis. Am J Pathol 2004; 164: 2139–2151.
Parikh AA, Liu WB, Fan F, Stoeltzing O, Reinmuth N, Bruns CJ et al. Expression and regulation of the novel vascular endothelial growth factor receptor neuropilin-1 by epidermal growth factor in human pancreatic carcinoma. Cancer 2003; 98: 720–729.
Vanveldhuizen PJ, Zulfiqar M, Banerjee S, Cherian R, Saxena NK, Rabe A et al. Differential expression of neuropilin-1 in malignant and benign prostatic stromal tissue. Oncol Rep 2003; 10: 1067–1071.
Kawakami T, Tokunaga T, Hatanaka H, Kijima H, Yamazaki H, Abe Y et al. Neuropilin 1 and neuropilin 2 co-expression is significantly correlated with increased vascularity and poor prognosis in nonsmall cell lung carcinoma. Cancer 2002; 95: 2196–2201.
Miao HQ, Lee P, Lin H, Soker S, Klagsbrun M . Neuropilin-1 expression by tumor cells promotes tumor angiogenesis and progression. FASEB J 2000; 14: 2532–2539.
Gray MJ, Wey JS, Belcheva A, McCarty MF, Trevino JG, Evans DB et al. Neuropilin-1 suppresses tumorigenic properties in a human pancreatic adenocarcinoma cell line lacking neuropilin-1 coreceptors. Cancer Res 2005; 65: 3664–3670.
Jia H, Cheng L, Tickner M, Bagherzadeh A, Selwood D, Zachary I . Neuropilin-1 antagonism in human carcinoma cells inhibits migration and enhances chemosensitivity. Br J Cancer 2010; 102: 541–552.
Li M, Yang H, Chai H, Fisher WE, Wang X, Brunicardi FC et al. Pancreatic carcinoma cells express neuropilins and vascular endothelial growth factor, but not vascular endothelial growth factor receptors. Cancer 2004; 101: 2341–2350.
Cao Y, G E, Wang E, Pal K, Dutta SK, Bar-Sagi D et al. VEGF exerts an angiogenesis-independent function in cancer cells to promote their malignant progression. Cancer Res 2012; 72: 3912–3918.
Lampropoulou A, Ruhrberg C . Neuropilin regulation of angiogenesis. Biochem Soc Trans 2014; 42: 1623–1628.
Chew YC, Adhikary G, Xu W, Wilson GM, Eckert RL . Protein kinase C delta increases kruppel-like factor 4 protein, which drives involucrin gene transcription in differentiating keratinocytes. J Biol Chem 2013; 288: 17759–17768.
Streit M, Velasco P, Brown LF, Skobe M, Richard L, Riccardi L et al. Overexpression of thrombospondin-1 decreases angiogenesis and inhibits the growth of human cutaneous squamous cell carcinomas. Am J Pathol 1999; 155: 441–452.
McCabe MT, Ott HM, Ganji G, Korenchuk S, Thompson C, Van Aller GS et al. EZH2 inhibition as a therapeutic strategy for lymphoma with EZH2-activating mutations. Nature 2012; 492: 108–112.
Acknowledgements
This work was supported by grants from the Maryland Stem Cell Research Foundation (RLE) and the National Institutes of Health (RLE - CA131074, CA184027) and a pilot grant from the Greenebaum Cancer Center (P30 CA134274).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Grun, D., Adhikary, G. & Eckert, R. VEGF-A acts via neuropilin-1 to enhance epidermal cancer stem cell survival and formation of aggressive and highly vascularized tumors. Oncogene 35, 4379–4387 (2016). https://doi.org/10.1038/onc.2015.507
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2015.507
This article is cited by
-
VEGF/Nrp1/HIF-1α promotes proliferation of hepatocellular carcinoma through a positive feedback loop
Medical Oncology (2023)
-
Endothelial Glycocalyx-Mediated Intercellular Interactions: Mechanisms and Implications for Atherosclerosis and Cancer Metastasis
Cardiovascular Engineering and Technology (2021)
-
Immunosuppression in Medulloblastoma: Insights into Cancer Immunity and Immunotherapy
Current Treatment Options in Oncology (2021)
-
ACTL6A suppresses p21Cip1 expression to enhance the epidermal squamous cell carcinoma phenotype
Oncogene (2020)
-
LncRNA TTN-AS1 promotes the progression of cholangiocarcinoma via the miR-320a/neuropilin-1 axis
Cell Death & Disease (2020)