Abstract
The SOCS1 gene coding for suppressor of cytokine signaling 1 is frequently repressed in hepatocellular carcinoma (HCC), and hence SOCS1 is considered a tumor suppressor in the liver. However, the tumor-suppressor mechanisms of SOCS1 are not yet well understood. SOCS1 is known to inhibit pro-inflammatory cytokine production and signaling and to promote activation of the p53 tumor suppressor. However, we observed that SOCS1-deficient mice developed numerous and large liver tumor nodules following treatment with the hepatocarcinogen diethylnitrosamine (DEN) without showing increased interleukin-6 production or activation of p53. On the other hand, the livers of DEN-treated Socs1-null mice showed elevated levels of p21CIP1/WAF1 protein (p21). Even though p21 generally functions as a tumor suppressor, paradoxically many cancers, including HCC, are known to express elevated levels of p21 that correlate with poor prognosis. We observed elevated p21 expression also in the regenerating livers of SOCS1-deficient mice and in cisplatin-treated Socs1-null hepatocytes, wherein the p21 protein showed increased stability. We show that SOCS1 interacts with p21 and promotes its ubiquitination and proteasomal degradation. Besides, the DEN-treated livers of Socs1-null mice showed increased nuclear and cytosolic p21 staining, and the latter was associated with growth factor-induced, phosphatidylinositol 3-kinase-dependent phosphorylation of p21 in SOCS1-deficient hepatocytes. Cytosolic p21 is often associated with malignancy and chemo-resistance in many cancers. Accordingly, SOCS1-deficient hepatocytes showed increased resistance to apoptosis that was reversed by shRNA-mediated p21 knockdown. In the regenerating livers of Socs1-null mice, increased p21 expression coincided with elevated cyclinD levels. Correspondingly, SOCS1-deficient hepatocytes showed increased proliferation to growth factor stimulation that was reversed by p21 knockdown. Overall, our findings indicate that the tumor-suppressor functions of SOCS1 in the liver could be mediated, at least partly, via regulation of the expression, stability and subcellular distribution of p21 and its paradoxical oncogenic functions, namely, resistance to apoptosis and increased proliferation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout









Similar content being viewed by others
References
El-Serag HB . Hepatocellular carcinoma. N Engl J Med 2011; 365: 1118–1127.
Lachenmayer A, Alsinet C, Chang CY, Llovet JM . Molecular approaches to treatment of hepatocellular carcinoma. Dig Liver Dis 2010; 42 (Suppl 3): S264–S272.
Farazi PA, DePinho RA . Hepatocellular carcinoma pathogenesis: from genes to environment. Nat Rev Cancer 2006; 6: 674–687.
Yoshikawa H, Matsubara K, Qian GS, Jackson P, Groopman JD, Manning JE et al. SOCS-1, a negative regulator of the JAK/STAT pathway, is silenced by methylation in human hepatocellular carcinoma and shows growth-suppression activity. Nat Genet 2001; 28: 29–35.
Yoshida T, Ogata H, Kamio M, Joo A, Shiraishi H, Tokunaga Y et al. SOCS1 is a suppressor of liver fibrosis and hepatitis-induced carcinogenesis. J Exp Med 2004; 199: 1701–1707.
Yoshimura A, Naka T, Kubo M . SOCS proteins, cytokine signalling and immune regulation. Nat Rev Immunol 2007; 7: 454–465.
Kazi JU, Kabir NN, Flores-Morales A, Ronnstrand L . SOCS proteins in regulation of receptor tyrosine kinase signaling. Cell Mol Life Sci 2014; 71: 3297–3310.
Marine JC, Topham DJ, McKay C, Wang D, Parganas E, Stravopodis D et al. SOCS1 deficiency causes a lymphocyte-dependent perinatal lethality. Cell 1999; 98: 609–616.
Taub R . Liver regeneration: from myth to mechanism. Nat Rev Mol Cell Biol 2004; 5: 836–847.
Naugler WE, Sakurai T, Kim S, Maeda S, Kim K, Elsharkawy AM et al. Gender disparity in liver cancer due to sex differences in MyD88-dependent IL-6 production. Science 2007; 317: 121–124.
Gui Y, Yeganeh M, Ramanathan S, Leblanc C, Pomerleau V, Ferbeyre G et al. SOCS1 controls liver regeneration by regulating HGF signaling in hepatocytes. J Hepatol 2011; 55: 1300–1308.
Calabrese V, Mallette FA, Deschenes-Simard X, Ramanathan S, Gagnon J, Moores A et al. SOCS1 links cytokine signaling to p53 and senescence. Mol Cell 2009; 36: 754–767.
Gartel AL, Tyner AL . The role of the cyclin-dependent kinase inhibitor p21 in apoptosis. Mol Cancer Ther 2002; 1: 639–649.
Gartel AL . Is p21 an oncogene? Mol Cancer Ther 2006; 5: 1385–1386.
Gartel AL . p21(WAF1/CIP1) and cancer: a shifting paradigm? Biofactors 2009; 35: 161–164.
Abbas T, Dutta A . p21 in cancer: intricate networks and multiple activities. Nat Rev Cancer 2009; 9: 400–414.
Warfel NA, El-Deiry WS . p21WAF1 and tumourigenesis: 20 years after. Curr Opin Oncol 2013; 25: 52–58.
Matsuda M, Nakamoto Y, Suzuki S, Kurata T, Kaneko S . Interferon-gamma-mediated hepatocarcinogenesis in mice treated with diethylnitrosamine. Lab Invest 2005; 85: 655–663.
Park EJ, Lee JH, Yu GY, He G, Ali SR, Holzer RG et al. Dietary and genetic obesity promote liver inflammation and tumorigenesis by enhancing IL-6 and TNF expression. Cell 2010; 140: 197–208.
Budhu A, Wang XW . The role of cytokines in hepatocellular carcinoma. J Leukoc Biol 2006; 80: 1197–1213.
Starostina NG, Kipreos ET . Multiple degradation pathways regulate versatile CIP/KIP CDK inhibitors. Trends Cell Biol 2012; 22: 33–41.
Okumura F, Matsuzaki M, Nakatsukasa K, Kamura T . The Role of elongin BC-containing ubiquitin ligases. Front Oncol 2012; 2: 10.
Woelk T, Sigismund S, Penengo L, Polo S . The ubiquitination code: a signalling problem. Cell Div 2007; 2: 11.
De la Cueva E, Garcia-Cao I, Herranz M, Lopez P, Garcia-Palencia P, Flores JM et al. Tumorigenic activity of p21Waf1/Cip1 in thymic lymphoma. Oncogene 2006; 25: 4128–4132.
Zhou BP, Liao Y, Xia W, Spohn B, Lee MH, Hung MC . Cytoplasmic localization of p21Cip1/WAF1 by Akt-induced phosphorylation in HER-2/neu-overexpressing cells. Nat Cell Biol 2001; 3: 245–252.
Natarajan A, Wagner B, Sibilia M . The EGF receptor is required for efficient liver regeneration. Proc Natl Acad Sci USA 2007; 104: 17081–17086.
Wierod L, Rosseland CM, Lindeman B, Oksvold MP, Grosvik H, Skarpen E et al. Activation of the p53-p21(Cip1) pathway is required for CDK2 activation and S-phase entry in primary rat hepatocytes. Oncogene 2008; 27: 2763–2771.
Skarpen E, Oksvold MP, Grosvik H, Widnes C, Huitfeldt HS . Altered regulation of EGF receptor signaling following a partial hepatectomy. J Cell Physiol 2005; 202: 707–716.
Dash BC, El-Deiry WS . Phosphorylation of p21 in G2/M promotes cyclin B-Cdc2 kinase activity. Mol Cell Biol 2005; 25: 3364–3387.
Anderson P . Post-transcriptional regulons coordinate the initiation and resolution of inflammation. Nat Rev Immunol 2010; 10: 24–35.
Iwahori K, Serada S, Fujimoto M, Ripley B, Nomura S, Mizuguchi H et al. SOCS-1 gene delivery cooperates with cisplatin plus pemetrexed to exhibit preclinical antitumor activity against malignant pleural mesothelioma. Int J Cancer 2013; 132: 459–471.
Kong X, Feng D, Wang H, Hong F, Bertola A, Wang FS et al. Interleukin-22 induces hepatic stellate cell senescence and restricts liver fibrosis in mice. Hepatology 2012; 56: 1150–1159.
Palmero I, Pantoja C, Serrano M . p19ARF links the tumour suppressor p53 to Ras. Nature 1998; 395: 125–126.
Velimezi G, Liontos M, Vougas K, Roumeliotis T, Bartkova J, Sideridou M et al. Functional interplay between the DNA-damage-response kinase ATM and ARF tumour suppressor protein in human cancer. Nat Cell Biol 2013; 15: 967–977.
Schneller D, Machat G, Sousek A, Proell V, van Zijl F, Zulehner G et al. p19(ARF) /p14(ARF) controls oncogenic functions of signal transducer and activator of transcription 3 in hepatocellular carcinoma. Hepatology 2011; 54: 164–172.
Gui Y, Yeganeh M, Donates YC, Tobelaim WS, Chababi W, Mayhue M et al. Regulation of MET receptor tyrosine kinase signaling by suppressor of cytokine signaling 1 in hepatocellular carcinoma. Oncogene 2015; 34: 5718–5728.
Seki E, Kondo Y, Iimuro Y, Naka T, Son G, Kishimoto T et al. Demonstration of cooperative contribution of MET- and EGFR-mediated STAT3 phosphorylation to liver regeneration by exogenous suppressor of cytokine signalings. J Hepatol 2008; 48: 237–245.
Boylan JM, Gruppuso PA . D-type cyclins and G1 progression during liver development in the rat. Biochem Biophys Res Commun 2005; 330: 722–730.
Rickheim DG, Nelsen CJ, Fassett JT, Timchenko NA, Hansen LK, Albrecht JH . Differential regulation of cyclins D1 and D3 in hepatocyte proliferation. Hepatology 2002; 36: 30–38.
Sheng G, Bernabe KQ, Guo J, Warner BW . Epidermal growth factor receptor-mediated proliferation of enterocytes requires p21waf1/cip1 expression. Gastroenterology 2006; 131: 153–164.
Buitrago-Molina LE, Marhenke S, Longerich T, Sharma AD, Boukouris AE, Geffers R et al. The degree of liver injury determines the role of p21 in liver regeneration and hepatocarcinogenesis in mice. Hepatology 2013; 58: 1143–1152.
Marhenke S, Buitrago-Molina LE, Endig J, Orlik J, Schweitzer N, Klett S et al. p21 promotes sustained liver regeneration and hepatocarcinogenesis in chronic cholestatic liver injury. Gut 2014; 63: 1501–1512.
Oh J, Hur MW, Lee CE . SOCS1 protects protein tyrosine phosphatases by thioredoxin upregulation and attenuates Jaks to suppress ROS-mediated apoptosis. Oncogene 2009; 28: 3145–3156.
Chen W, Sun Z, Wang XJ, Jiang T, Huang Z, Fang D et al. Direct interaction between Nrf2 and p21(Cip1/WAF1) upregulates the Nrf2-mediated antioxidant response. Mol Cell 2009; 34: 663–673.
Hui L, Zatloukal K, Scheuch H, Stepniak E, Wagner EF . Proliferation of human HCC cells and chemically induced mouse liver cancers requires JNK1-dependent p21 downregulation. J Clin Invest 2008; 118: 3943–3953.
Tanaka K, Ichiyama K, Hashimoto M, Yoshida H, Takimoto T, Takaesu G et al. Loss of suppressor of cytokine signaling 1 in helper T cells leads to defective Th17 differentiation by enhancing antagonistic effects of IFN-gamma on STAT3 and Smads. J Immunol 2008; 180: 3746–3756.
Acknowledgements
MY was a recipient of FRQNT graduate scholarship. YG was supported by a graduate scholarship from the Canadian Liver Foundation. RK is supported by a graduate scholarship from the Faculté de médecine et des sciences de la santé, Université de Sherbrooke. DB is a recipient of a postdoctoral fellowship from FRQS. CR-CHUS is an FRQS-funded research center. This work was supported by operating grants from the Canadian Institutes of Heath research (to SI, MOP-84234) and Cancer Research Society, Montreal, Canada (to SI and GF, PIN: 17009).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Yeganeh, M., Gui, Y., Kandhi, R. et al. Suppressor of cytokine signaling 1-dependent regulation of the expression and oncogenic functions of p21CIP1/WAF1 in the liver. Oncogene 35, 4200–4211 (2016). https://doi.org/10.1038/onc.2015.485
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2015.485
This article is cited by
-
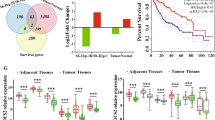
Prognostic significance of SOCS1 and SOCS3 tumor suppressors and oncogenic signaling pathway genes in hepatocellular carcinoma
BMC Cancer (2020)
-
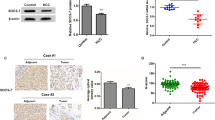
HJURP promotes hepatocellular carcinoma proliferation by destabilizing p21 via the MAPK/ERK1/2 and AKT/GSK3β signaling pathways
Journal of Experimental & Clinical Cancer Research (2018)
-
Expression of SOCS1 and the downstream targets of its putative tumor suppressor functions in prostate cancer
BMC Cancer (2017)
-
SOCS1 inhibits migration and invasion of prostate cancer cells, attenuates tumor growth and modulates the tumor stroma
Prostate Cancer and Prostatic Diseases (2017)