Abstract
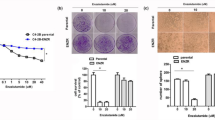
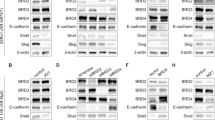
PI3K (phosphoinositide 3-kinase)/AKT and RAS/MAPK (mitogen-activated protein kinase) pathway coactivation in the prostate epithelium promotes both epithelial–mesenchymal transition (EMT) and metastatic castration-resistant prostate cancer (mCRPC), which is currently incurable. To study the dynamic regulation of the EMT process, we developed novel genetically defined cellular and in vivo model systems from which epithelial, EMT and mesenchymal-like tumor cells with Pten deletion and Kras activation can be isolated. When cultured individually, each population has the capacity to regenerate all three tumor cell populations, indicative of epithelial–mesenchymal plasticity. Despite harboring the same genetic alterations, mesenchymal-like tumor cells are resistant to PI3K and MAPK pathway inhibitors, suggesting that epigenetic mechanisms may regulate the EMT process, as well as dictate the heterogeneous responses of cancer cells to therapy. Among differentially expressed epigenetic regulators, the chromatin remodeling protein HMGA2 is significantly upregulated in EMT and mesenchymal-like tumors cells, as well as in human mCRPC. Knockdown of HMGA2, or suppressing HMGA2 expression with the histone deacetylase inhibitor LBH589, inhibits epithelial–mesenchymal plasticity and stemness activities in vitro and markedly reduces tumor growth and metastasis in vivo through successful targeting of EMT and mesenchymal-like tumor cells. Importantly, LBH589 treatment in combination with castration prevents mCRPC development and significantly prolongs survival following castration by enhancing p53 and androgen receptor acetylation and in turn sensitizing castration-resistant mesenchymal-like tumor cells to androgen deprivation therapy. Taken together, these findings demonstrate that cellular plasticity is regulated epigenetically, and that mesenchymal-like tumor cell populations in mCRPC that are resistant to conventional and targeted therapies can be effectively treated with the epigenetic inhibitor LBH589.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Accession codes
References
Siegel RL, Miller KD, Jemal A . Cancer statistics, 2015. Cancer J Clin 2015; 65: 5–29.
Attard G, de Bono JS . Translating scientific advancement into clinical benefit for castration-resistant prostate cancer patients. Clin Cancer Res 2011; 17: 3867–3875.
de Bono JS, Logothetis CJ, Molina A, Fizazi K, North S, Chu L et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med 2011; 364: 1995–2005.
Scher HI, Fizazi K, Saad F, Taplin ME, Sternberg CN, Miller K et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med 2012; 367: 1187–1197.
Rescigno P, Buonerba C, Bellmunt J, Sonpavde G, De Placido S, Di Lorenzo G . New perspectives in the therapy of castration resistant prostate cancer. Curr Drug Targets 2012; 13: 1676–1686.
Shah RB, Mehra R, Chinnaiyan AM, Shen R, Ghosh D, Zhou M et al. Androgen-independent prostate cancer is a heterogeneous group of diseases: lessons from a rapid autopsy program. Cancer Res 2004; 64: 9209–9216.
Taylor BS, Schultz N, Hieronymus H, Gopalan A, Xiao Y, Carver BS et al. Integrative genomic profiling of human prostate cancer. Cancer Cell 2010; 18: 11–22.
Baca SC, Garraway LA . The genomic landscape of prostate cancer. Front Endocrinol 2012; 3: 69.
Brannon AR, Sawyers CL . ‘N of 1’ case reports in the era of whole-genome sequencing. J Clin Invest 2013; 123: 4568–4570.
Haffner MC, Mosbruger T, Esopi DM, Fedor H, Heaphy CM, Walker DA et al. Tracking the clonal origin of lethal prostate cancer. J Clin Invest 2013; 123: 4918–4922.
Tanaka H, Kono E, Tran CP, Miyazaki H, Yamashiro J, Shimomura T et al. Monoclonal antibody targeting of N-cadherin inhibits prostate cancer growth, metastasis and castration resistance. Nat Med 2010; 16: 1414–1420.
Sun Y, Wang BE, Leong KG, Yue P, Li L, Jhunjhunwala S et al. Androgen deprivation causes epithelial–mesenchymal transition in the prostate: implications for androgen-deprivation therapy. Cancer Res 2012; 72: 527–536.
Armstrong AJ, Marengo MS, Oltean S, Kemeny G, Bitting RL, Turnbull JD et al. Circulating tumor cells from patients with advanced prostate and breast cancer display both epithelial and mesenchymal markers. Mol Cancer Res 2011; 9: 997–1007.
Bitting RL, Schaeffer D, Somarelli JA, Garcia-Blanco MA, Armstrong AJ . The role of epithelial plasticity in prostate cancer dissemination and treatment resistance. Cancer Metast Rev 2014; 33: 441–468.
Marin-Aguilera M, Codony-Servat J, Reig O, Lozano JJ, Fernandez PL, Pereira MV et al. Epithelial-to-mesenchymal transition mediates docetaxel resistance and high risk of relapse in prostate cancer. Mol Cancer Therap 2014; 13: 1270–1284.
Mulholland DJ, Kobayashi N, Ruscetti M, Zhi A, Tran LM, Huang J et al. Pten loss and RAS/MAPK activation cooperate to promote EMT and metastasis initiated from prostate cancer stem/progenitor cells. Cancer Res 2012; 72: 1878–1889.
Ruscetti M, Quach B, Dadashian EL, Mulholland DJ, Wu H . Tracking and functional characterization of epithelial–mesenchymal transition and mesenchymal tumor cells during prostate cancer metastasis. Cancer Res 2015; 75: 2149–2159.
Grasso CS, Wu YM, Robinson DR, Cao X, Dhanasekaran SM, Khan AP et al. The mutational landscape of lethal castration-resistant prostate cancer. Nature 2012; 487: 239–243.
Tam WL, Weinberg RA . The epigenetics of epithelial–mesenchymal plasticity in cancer. Nat Med 2013; 19: 1438–1449.
Fusco A, Fedele M . Roles of HMGA proteins in cancer. Nat Rev Cancer 2007; 7: 899–910.
Nishino J, Kim I, Chada K, Morrison SJ . Hmga2 promotes neural stem cell self-renewal in young but not old mice by reducing p16Ink4a and p19Arf Expression. Cell 2008; 135: 227–239.
Li O, Vasudevan D, Davey CA, Droge P . High-level expression of DNA architectural factor HMGA2 and its association with nucleosomes in human embryonic stem cells. Genesis 2006; 44: 523–529.
Rommel B, Rogalla P, Jox A, Kalle CV, Kazmierczak B, Wolf J et al. HMGI-C, a member of the high mobility group family of proteins, is expressed in hematopoietic stem cells and in leukemic cells. Leuk Lymphoma 1997; 26: 603–607.
Zong Y, Huang J, Sankarasharma D, Morikawa T, Fukayama M, Epstein JI et al. Stromal epigenetic dysregulation is sufficient to initiate mouse prostate cancer via paracrine Wnt signaling. Proc Natl Acad Sci USA 2012; 109: E3395–E3404.
Watanabe S, Ueda Y, Akaboshi S, Hino Y, Sekita Y, Nakao M . HMGA2 maintains oncogenic RAS-induced epithelial–mesenchymal transition in human pancreatic cancer cells. Am J Pathol 2009; 174: 854–868.
Luo Y, Li W, Liao H . HMGA2 induces epithelial-to-mesenchymal transition in human hepatocellular carcinoma cells. Oncol Lett 2013; 5: 1353–1356.
Sun M, Song CX, Huang H, Frankenberger CA, Sankarasharma D, Gomes S et al. HMGA2/TET1/HOXA9 signaling pathway regulates breast cancer growth and metastasis. Proc Natl Acad Sci USA 2013; 110: 9920–9925.
Zha L, Wang Z, Tang W, Zhang N, Liao G, Huang Z . Genome-wide analysis of HMGA2 transcription factor binding sites by ChIP on chip in gastric carcinoma cells. Mol Cell Biochem 2012; 364: 243–251.
Gu L, Frommel SC, Oakes CC, Simon R, Grupp K, Gerig CY et al. BAZ2A (TIP5) is involved in epigenetic alterations in prostate cancer and its overexpression predicts disease recurrence. Nat Genet 2015; 47: 22–30.
Ferguson M, Henry PA, Currie RA . Histone deacetylase inhibition is associated with transcriptional repression of the Hmga2 gene. Nucleic Acids Res 2003; 31: 3123–3133.
Lee S, Jung JW, Park SB, Roh K, Lee SY, Kim JH et al. Histone deacetylase regulates high mobility group A2-targeting microRNAs in human cord blood-derived multipotent stem cell aging. Cell Mol Life Sci 2011; 68: 325–336.
Luo J, Li M, Tang Y, Laszkowska M, Roeder RG, Gu W . Acetylation of p53 augments its site-specific DNA binding both in vitro and in vivo. Proc Natl Acad Sci USA 2004; 101: 2259–2264.
Gu W, Roeder RG . Activation of p53 sequence-specific DNA binding by acetylation of the p53 C-terminal domain. Cell 1997; 90: 595–606.
Lei Q, Jiao J, Xin L, Chang CJ, Wang S, Gao J et al. NKX3.1 stabilizes p53, inhibits AKT activation, and blocks prostate cancer initiation caused by PTEN loss. Cancer Cell 2006; 9: 367–378.
Wang S, Gao J, Lei Q, Rozengurt N, Pritchard C, Jiao J et al. Prostate-specific deletion of the murine Pten tumor suppressor gene leads to metastatic prostate cancer. Cancer Cell 2003; 4: 209–221.
Mulholland DJ, Xin L, Morim A, Lawson D, Witte O, Wu H . Lin-Sca-1+CD49fhigh stem/progenitors are tumor-initiating cells in the Pten-null prostate cancer model. Cancer Res 2009; 69: 8555–8562.
Fu M, Rao M, Wang C, Sakamaki T, Wang J, Di Vizio D et al. Acetylation of androgen receptor enhances coactivator binding and promotes prostate cancer cell growth. Mol Cell Biol 2003; 23: 8563–8575.
Haelens A, Tanner T, Denayer S, Callewaert L, Claessens F . The hinge region regulates DNA binding, nuclear translocation, and transactivation of the androgen receptor. Cancer Res 2007; 67: 4514–4523.
Gaughan L, Logan IR, Cook S, Neal DE, Robson CN . Tip60 and histone deacetylase 1 regulate androgen receptor activity through changes to the acetylation status of the receptor. J Biol Chem 2002; 277: 25904–25913.
Muranen T, Selfors LM, Worster DT, Iwanicki MP, Song L, Morales FC et al. Inhibition of PI3K/mTOR leads to adaptive resistance in matrix-attached cancer cells. Cancer Cell 2012; 21: 227–239.
Konieczkowski DJ, Johannessen CM, Abudayyeh O, Kim JW, Cooper ZA, Piris A et al. A melanoma cell state distinction influences sensitivity to MAPK pathway inhibitors. Cancer Discov 2014; 4: 816–827.
Rathkopf D, Wong BY, Ross RW, Anand A, Tanaka E, Woo MM et al. A phase I study of oral panobinostat alone and in combination with docetaxel in patients with castration-resistant prostate cancer. Cancer Chemother Pharmacol 2010; 66: 181–189.
Molife LR, Attard G, Fong PC, Karavasilis V, Reid AH, Patterson S et al. Phase II, two-stage, single-arm trial of the histone deacetylase inhibitor (HDACi) romidepsin in metastatic castration-resistant prostate cancer (CRPC). Ann Oncol 2010; 21: 109–113.
Bradley D, Rathkopf D, Dunn R, Stadler WM, Liu G, Smith DC et al. Vorinostat in advanced prostate cancer patients progressing on prior chemotherapy (National Cancer Institute Trial 6862): trial results and interleukin-6 analysis: a study by the Department of Defense Prostate Cancer Clinical Trial Consortium and University of Chicago Phase 2 Consortium. Cancer 2009; 115: 5541–5549.
Rathkopf DE, Picus J, Hussain A, Ellard S, Chi KN, Nydam T et al. A phase 2 study of intravenous panobinostat in patients with castration-resistant prostate cancer. Cancer Chemother Pharmacol 2013; 72: 537–544.
Welsbie DS, Xu J, Chen Y, Borsu L, Scher HI, Rosen N et al. Histone deacetylases are required for androgen receptor function in hormone-sensitive and castrate-resistant prostate cancer. Cancer Res 2009; 69: 958–966.
Liu X, Gomez-Pinillos A, Liu X, Johnson EM, Ferrari AC . Induction of bicalutamide sensitivity in prostate cancer cells by an epigenetic Puralpha-mediated decrease in androgen receptor levels. Prostate 2010; 70: 179–189.
Langelotz C, Schmid P, Jakob C, Heider U, Wernecke KD, Possinger K et al. Expression of high-mobility-group-protein HMGI-C mRNA in the peripheral blood is an independent poor prognostic indicator for survival in metastatic breast cancer. Br J Cancer 2003; 88: 1406–1410.
Winslow MM, Dayton TL, Verhaak RG, Kim-Kiselak C, Snyder EL, Feldser DM et al. Suppression of lung adenocarcinoma progression by Nkx2-1. Nature 2011; 473: 101–104.
Sarbassov DD, Guertin DA, Ali SM, Sabatini DM . Phosphorylation and regulation of Akt/PKB by the rictor–mTOR complex. Science 2005; 307: 1098–1101.
Acknowledgements
We thank members of the Hong Wu lab for their critical comments and suggestions. We also thank Drs Yang Zong and Owen Witte for generously supplying us with HMGA2 antibodies, and Dr Shumin Wu for supplying protein lysates from mouse ES cells. MR was supported by NIH T32 CA009056. WG was supported by a General Financial Grant from the China Postdoctoral Science Foundation (2015M570010), and in part by the Postdoctoral Fellowship of Peking-Tsinghua Center for Life Sciences. DJM was supported by NIH F32 CA112988-01, CIRM TG2-01169 and a Prostate Cancer Foundation Young Investigator Award. This work has been supported, in part, by awards from the Prostate Cancer Foundation (to HW), and grants from the NIH (P50 CA097186 and P01 CA163227 to PSN, P50 CA092131 to YX and HW, and R01 CA107166, RO1 CA121110 and U01 CA164188 to HW).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Ruscetti, M., Dadashian, E., Guo, W. et al. HDAC inhibition impedes epithelial–mesenchymal plasticity and suppresses metastatic, castration-resistant prostate cancer. Oncogene 35, 3781–3795 (2016). https://doi.org/10.1038/onc.2015.444
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2015.444
This article is cited by
-
SYTL2 promotes metastasis of prostate cancer cells by enhancing FSCN1-mediated pseudopodia formation and invasion
Journal of Translational Medicine (2023)
-
The epithelial–mesenchymal plasticity landscape: principles of design and mechanisms of regulation
Nature Reviews Genetics (2023)
-
S100PBP is regulated by mutated KRAS and plays a tumour suppressor role in pancreatic cancer
Oncogene (2023)
-
Regulation of epithelial-mesenchymal transition by protein lysine acetylation
Cell Communication and Signaling (2022)
-
Targeting cancer stem cells with polymer nanoparticles for gastrointestinal cancer treatment
Stem Cell Research & Therapy (2022)