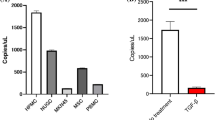
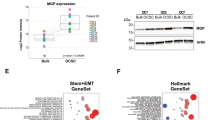
Abstract
The cross-talk between ovarian cancer (OvCa) cells and the metastatic microenvironment is an essential determinant of successful colonization. MicroRNAs (miRNAs) have several critical roles during metastasis; however, the role of microenvironmental cues in the regulation of miRNAs in metastasizing cancer cells has not been studied. Using a three-dimensional culture model that mimics the human omentum, one of the principal sites of OvCa metastasis, we identified and characterized the microenvironment-induced downregulation of a tumor suppressor miRNA, miR-193b, in metastasizing OvCa cells. The direct interaction of the OvCa cells with mesothelial cells, which cover the surface of the omentum, caused a DNA methyltransferase 1-mediated decrease in the expression of miR-193b in the cancer cells. The reduction in miR-193b enabled the metastasizing cancer cells to invade and proliferate into human omental pieces ex vivo and into the omentum of a mouse xenograft model of OvCa metastasis. The functional effects of miR-193b were mediated, in large part, by the concomitant increased expression of its target, urokinase-type plasminogen activator, a known tumor-associated protease. These findings link paracrine signals from the microenvironment to the regulation of a key miRNA in cancer cells. Targeting miR-193b, which is essential for metastatic colonization of cancer cells could prove effective in the treatment of OvCa metastasis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Vaughan S, Coward JI, Bast RC Jr, Berchuck A, Berek JS, Brenton JD et al. Rethinking ovarian cancer: recommendations for improving outcomes. Nat Rev Cancer 2011; 11: 719–725.
Bast RC Jr, Hennessy B, Mills GB . The biology of ovarian cancer: new opportunities for translation. Nat Rev Cancer 2009; 9: 415–428.
Lengyel E . Ovarian cancer development and metastasis. Am J Pathol 2010; 177: 1053–1064.
Chambers AF, Groom AC, MacDonald IC . Dissemination and growth of cancer cells in metastatic sites. Nat Rev Cancer 2002; 2: 563–572.
Kenny HA, Chiang CY, White EA, Schryver EM, Habis M, Romero IL et al. Mesothelial cells promote early ovarian cancer metastasis through fibronectin secretion. J Clin Invest 2014; 124: 4614–4628.
Lujambio A, Lowe SW . The microcosmos of cancer. Nature 2012; 482: 347–355.
Pencheva N, Tavazoie SF . Control of metastatic progression by microRNA regulatory networks. Nat Cell Biol 2013; 15: 546–554.
Ma L, Teruya-Feldstein J, Weinberg RA . Tumour invasion and metastasis initiated by microRNA-10b in breast cancer. Nature 2007; 449: 682–688.
Huang Q, Gumireddy K, Schrier M, le Sage C, Nagel R, Nair S et al. The microRNAs miR-373 and miR-520c promote tumour invasion and metastasis. Nat Cell Biol 2008; 10: 202–210.
Cai J, Guan H, Fang L, Yang Y, Zhu X, Yuan J et al. MicroRNA-374a activates Wnt/beta-catenin signaling to promote breast cancer metastasis. J Clin Invest 2013; 123: 566–579.
Song SJ, Poliseno L, Song MS, Ala U, Webster K, Ng C et al. MicroRNA-antagonism regulates breast cancer stemness and metastasis via TET-family-dependent chromatin remodeling. Cell 2013; 154: 311–324.
Nicoli S, Standley C, Walker P, Hurlstone A, Fogarty KE, Lawson ND . MicroRNA-mediated integration of haemodynamics and Vegf signalling during angiogenesis. Nature 2010; 464: 1196–1200.
Lwin T, Zhao X, Cheng F, Zhang X, Huang A, Shah B et al. A microenvironment-mediated c-Myc/miR-548 m/HDAC6 amplification loop in non-Hodgkin B cell lymphomas. J Clin Invest 2013; 123: 4612–4626.
Mitra AK, Zillhardt M, Hua Y, Tiwari P, Murmann AE, Peter ME et al. MicroRNAs reprogram normal fibroblasts into cancer-associated fibroblasts in ovarian cancer. Cancer Discov 2012; 2: 1100–1108.
Hanahan D, Coussens LM . Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell 2012; 21: 309–322.
Kenny HA, Krausz T, Yamada SD, Lengyel E . Use of a novel 3D culture model to elucidate the role of mesothelial cells, fibroblasts and extra-cellular matrices on adhesion and invasion of ovarian cancer cells to the omentum. Int J Cancer 2007; 121: 1463–1472.
Zhang L, Volinia S, Bonome T, Calin GA, Greshock J, Yang N et al. Genomic and epigenetic alterations deregulate microRNA expression in human epithelial ovarian cancer. Proc Natl Acad Sci USA 2008; 105: 7004–7009.
Niedbala MJ, Crickard K, Bernacki RJ . Interactions of human ovarian tumor cells with human mesothelial cells grown on extracellular matrix. An in vitro model system for studying tumor cell adhesion and invasion. Exp Cell Res 1985; 160: 499–513.
Iwanicki MP, Davidowitz RA, Ng MR, Besser A, Muranen T, Merritt M et al. Ovarian cancer spheroids use myosin-generated force to clear the mesothelium. Cancer Discov 2011; 1: 144–157.
Mitra AK, Sawada K, Tiwari P, Mui K, Gwin K, Lengyel E . Ligand-independent activation of c-Met by fibronectin and alpha(5)beta(1)-integrin regulates ovarian cancer invasion and metastasis. Oncogene 2011; 30: 1566–1576.
Sawada K, Mitra AK, Radjabi AR, Bhaskar V, Kistner EO, Tretiakova M et al. Loss of E-cadherin promotes ovarian cancer metastasis via alpha 5-integrin, which is a therapeutic target. Cancer Res 2008; 68: 2329–2339.
Ameres SL, Zamore PD . Diversifying microRNA sequence and function. Nat Rev Mol Cell Biol 2013; 14: 475–488.
Goll MG, Bestor TH . Eukaryotic cytosine methyltransferases. Annu Rev Biochem 2005; 74: 481–514.
Schmalfeldt B, Prechtel D, Harting K, Spathe K, Rutke S, Konik E et al. Increased expression of matrix metalloproteinases (MMP)-2, MMP-9, and the urokinase-type plasminogen activator is associated with progression from benign to advanced ovarian cancer. Clin Cancer Res 2001; 7: 2396–2404.
Wang L, Madigan MC, Chen H, Liu F, Patterson KI, Beretov J et al. Expression of urokinase plasminogen activator and its receptor in advanced epithelial ovarian cancer patients. Gynecol Oncol 2009; 114: 265–272.
Zhang Y, Kenny HA, Swindell EP, Mitra AK, Hankins PL, Ahn RW et al. Urokinase plasminogen activator system-targeted delivery of nanobins as a novel ovarian cancer therapy. Mol Cancer Ther 2013; 12: 2628–2639.
Duffy MJ . The urokinase plasminogen activator system: role in malignancy. Curr Pharm Des 2004; 10: 39–49.
Valastyan S, Reinhardt F, Benaich N, Calogrias D, Szasz AM, Wang ZC et al. A pleiotropically acting microRNA, miR-31, inhibits breast cancer metastasis. Cell 2009; 137: 1032–1046.
Valastyan S, Weinberg RA . Tumor metastasis: molecular insights and evolving paradigms. Cell 2011; 147: 275–292.
Valiente M, Obenauf AC, Jin X, Chen Q, Zhang XH, Lee DJ et al. Serpins promote cancer cell survival and vascular co-option in brain metastasis. Cell 2014; 156: 1002–1016.
White EA, Kenny HA, Lengyel E . Three-dimensional modeling of ovarian cancer. Adv Drug Deliv Rev 2014; 79-80: 184–192.
Rauhala HE, Jalava SE, Isotalo J, Bracken H, Lehmusvaara S, Tammela TL et al. miR-193b is an epigenetically regulated putative tumor suppressor in prostate cancer. Int J Cancer 2010; 127: 1363–1372.
Gao XN, Lin J, Gao L, Li YH, Wang LL, Yu L . MicroRNA-193b regulates c-Kit proto-oncogene and represses cell proliferation in acute myeloid leukemia. Leuk Res 2011; 35: 1226–1232.
Xu C, Liu S, Fu H, Li S, Tie Y, Zhu J et al. MicroRNA-193b regulates proliferation, migration and invasion in human hepatocellular carcinoma cells. Eur J Cancer 2010; 46: 2828–2836.
Li XF, Yan PJ, Shao ZM . Downregulation of miR-193b contributes to enhance urokinase-type plasminogen activator (uPA) expression and tumor progression and invasion in human breast cancer. Oncogene 2009; 28: 3937–3948.
Lee JY, Jeong W, Lim W, Lim CH, Bae SM, Kim J et al. Hypermethylation and post-transcriptional regulation of DNA methyltransferases in the ovarian carcinomas of the laying hen. PLoS One 2013; 8: e61658.
Samudio-Ruiz SL, Hudson LG . Increased DNA methyltransferase activity and DNA methylation following epidermal growth factor stimulation in ovarian cancer cells. Epigenetics 2012; 7: 216–224.
Ahluwalia A, Hurteau JA, Bigsby RM, Nephew KP . DNA methylation in ovarian cancer. II. Expression of DNA methyltransferases in ovarian cancer cell lines and normal ovarian epithelial cells. Gynecol Oncol 2001; 82: 299–304.
Noh H, Hong S, Dong Z, Pan ZK, Jing Q, Huang S . Impaired microRNA processing facilitates breast cancer cell invasion by upregulating urokinase-type plasminogen activator expression. Genes Cancer 2011; 2: 140–150.
Xie C, Jiang XH, Zhang JT, Sun TT, Dong JD, Sanders AJ et al. CFTR suppresses tumor progression through miR-193b targeting urokinase plasminogen activator (uPA) in prostate cancer. Oncogene 2013; 32: 2291 e2281–2287.
Tang CH, Hill ML, Brumwell AN, Chapman HA, Wei Y . Signaling through urokinase and urokinase receptor in lung cancer cells requires interactions with beta1 integrins. J Cell Sci 2008; 121: 3747–3756.
Konecny G, Untch M, Pihan A, Kimmig R, Gropp M, Stieber P et al. Association of urokinase-type plasminogen activator and its inhibitor with disease progression and prognosis in ovarian cancer. Clin Cancer Res 2001; 7: 1743–1749.
Zhang W, Ling D, Tan J, Zhang J, Li L . Expression of urokinase plasminogen activator and plasminogen activator inhibitor type-1 in ovarian cancer and its clinical significance. Oncol Rep 2013; 29: 637–645.
Nieman KM, Romero IL, Van Houten B, Lengyel E . Adipose tissue and adipocytes support tumorigenesis and metastasis. Biochim Biophys Acta 2013; 1831: 1533–1541.
Brader KR, Wolf JK, Hung MC, Yu D, Crispens MA, van Golen KL et al. Adenovirus E1A expression enhances the sensitivity of an ovarian cancer cell line to multiple cytotoxic agents through an apoptotic mechanism. Clin Cancer Res 1997; 3: 2017–2024.
Nieman KM, Kenny HA, Penicka CV, Ladanyi A, Buell-Gutbrod R, Zillhardt MR et al. Adipocytes promote ovarian cancer metastasis and provide energy for rapid tumor growth. Nat Med 2011; 17: 1498–1503.
Acknowledgements
We want to thank Dr AF Haney for collecting omental biopsies, Dr Abir Mukherjee for helpful discussions and G Isenberg for carefully editing the manuscript (all at the University of Chicago, Department of Obstetrics and Gynecology). We are indebted to all the patients, resident and attending physicians in the Department of Obstetrics and Gynecology at the University of Chicago for their participation in tissue collection for these experiments. This research was supported by a Marsha Rivkin Pilot Award (AKM) and by National Cancer Institute R01 CA111882 and RO1 CA169604 grants (EL) and an Ovarian Cancer Research Fund Program Project Development Grant (EL, MEP).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Mitra, A., Chiang, C., Tiwari, P. et al. Microenvironment-induced downregulation of miR-193b drives ovarian cancer metastasis. Oncogene 34, 5923–5932 (2015). https://doi.org/10.1038/onc.2015.43
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2015.43
This article is cited by
-
The Potential of Circulating miR-193b, miR-146b-3p and miR-483-3p as Noninvasive Biomarkers in Cutaneous Melanoma Patients
Molecular Biotechnology (2023)
-
Molecular mediators of peritoneal metastasis in pancreatic cancer
Cancer and Metastasis Reviews (2020)
-
Current insights into the metastasis of epithelial ovarian cancer - hopes and hurdles
Cellular Oncology (2020)
-
The activation of microRNA-520h–associated TGF-β1/c-Myb/Smad7 axis promotes epithelial ovarian cancer progression
Cell Death & Disease (2018)
-
Malignant extracellular vesicles carrying MMP1 mRNA facilitate peritoneal dissemination in ovarian cancer
Nature Communications (2017)