Abstract
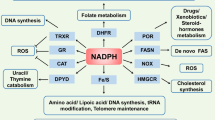
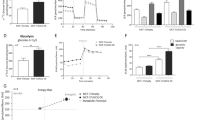
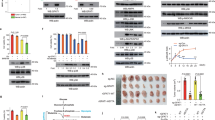
Nicotinamide phosphoribosyltransferase (NAMPT) is a rate-limiting enzyme involved in NAD+ biosynthesis. Although NAMPT has emerged as a critical regulator of metabolic stress, the underlying mechanisms by which it regulates metabolic stress in cancer cells have not been completely elucidated. In this study, we determined that breast cancer cells expressing a high level of NAMPT were resistant to cell death induced by glucose depletion. Furthermore, NAMPT inhibition suppressed tumor growth in vivo in a xenograft model. Under glucose deprivation conditions, NAMPT inhibition was found to increase the mitochondrial reactive oxygen species (ROS) level, leading to cell death. This cell death was rescued by treatment with antioxidants or NAD+. Finally, we showed that NAMPT increased the pool of NAD+ that could be converted to NADPH through the pentose phosphate pathway and inhibited the depletion of reduced glutathione under glucose deprivation. Collectively, our results suggest a novel mechanism by which tumor cells protect themselves against glucose deprivation-induced oxidative stress by utilizing NAMPT to maintain NADPH levels.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Cairns RA, Harris IS, Mak TW . Regulation of cancer cell metabolism. Nat Rev Cancer 2011; 11: 85–95.
Denko NC . Hypoxia, HIF1 and glucose metabolism in the solid tumour. Nat Rev Cancer 2008; 8: 705–713.
Sena LA, Chandel NS . Physiological roles of mitochondrial reactive oxygen species. Mol Cell 2012; 48: 158–167.
Li L, Ishdorj G, Gibson SB . Reactive oxygen species regulation of autophagy in cancer: implications for cancer treatment. Free Radic Biol Med 2012; 53: 1399–1410.
Trachootham D, Alexandre J, Huang P . Targeting cancer cells by ROS-mediated mechanisms: a radical therapeutic approach? Nat Rev Drug Discov 2009; 8: 579–591.
Ying W . NAD+/NADH and NADP+/NADPH in cellular functions and cell death: regulation and biological consequences. Antioxid Redox Signal 2008; 10: 179–206.
Madhok BM, Yeluri S, Perry SL, Hughes TA, Jayne DG . Targeting glucose metabolism: an emerging concept for anticancer therapy. Am J Clin Oncol 2011; 34: 628–635.
Kirkman HN, Gaetani GF . Catalase: a tetrameric enzyme with four tightly bound molecules of NADPH. Proc Natl Acad Sci USA 1984; 81: 4343–4347.
Chiarugi A, Dolle C, Felici R, Ziegler M . The NAD metabolome—a key determinant of cancer cell biology. Nat Rev Cancer 2012; 12: 741–752.
Garten A, Petzold S, Korner A, Imai S, Kiess W . Nampt: linking NAD biology, metabolism and cancer. Trends Endocrinol Metab 2009; 20: 130–138.
Yang H, Yang T, Baur JA, Perez E, Matsui T, Carmona JJ et al. Nutrient-sensitive mitochondrial NAD+ levels dictate cell survival. Cell 2007; 130: 1095–1107.
Wang B, Hasan MK, Alvarado E, Yuan H, Wu H, Chen WY . NAMPT overexpression in prostate cancer and its contribution to tumor cell survival and stress response. Oncogene 2011; 30: 907–921.
Bi TQ, Che XM . Nampt/PBEF/visfatin and cancer. Cancer Biol Ther 2010; 10: 119–125.
Galli U, Travelli C, Massarotti A, Fakhfouri G, Rahimian R, Tron GC et al. Medicinal chemistry of nicotinamide phosphoribosyltransferase (NAMPT) inhibitors. J Med Chem 2013; 56: 6279–6296.
Tan B, Young DA, Lu ZH, Wang T, Meier TI, Shepard RL et al. Pharmacological inhibition of nicotinamide phosphoribosyltransferase (NAMPT), an enzyme essential for NAD+ biosynthesis, in human cancer cells: metabolic basis and potential clinical implications. J Biol Chem 2013; 288: 3500–3511.
Simons AL, Mattson DM, Dornfeld K, Spitz DR . Glucose deprivation-induced metabolic oxidative stress and cancer therapy. J Cancer Res Ther 2009; 5: S2–S6.
Liang HL, Hilton G, Mortensen J, Regner K, Johnson CP, Nilakantan V . MnTMPyP, a cell-permeant SOD mimetic, reduces oxidative stress and apoptosis following renal ischemia-reperfusion. Am J Physiol Renal Physiol 2009; 296: F266–F276.
Romero-Garcia S, Lopez-Gonzalez JS, Baez-Viveros JL, Aguilar-Cazares D, Prado-Garcia H . Tumor cell metabolism: an integral view. Cancer Biol Ther 2011; 12: 939–948.
Tew KD, Townsend DM . Glutathione-s-transferases as determinants of cell survival and death. Antioxid Redox Signal 2012; 17: 1728–1737.
Aykin-Burns N, Ahmad IM, Zhu Y, Oberley LW, Spitz DR . Increased levels of superoxide and H2O2 mediate the differential susceptibility of cancer cells versus normal cells to glucose deprivation. Biochem J 2009; 418: 29–37.
Someya S, Yu W, Hallows WC, Xu J, Vann JM, Leeuwenburgh C et al. Sirt3 mediates reduction of oxidative damage and prevention of age-related hearing loss under caloric restriction. Cell 2010; 143: 802–812.
Magni G, Orsomando G, Raffaelli N . Structural and functional properties of NAD kinase, a key enzyme in NADP biosynthesis. Mini Rev Med Chem 2006; 6: 739–746.
Cerna D, Li H, Flaherty S, Takebe N, Coleman CN, Yoo SS . Inhibition of nicotinamide phosphoribosyltransferase (NAMPT) activity by small molecule GMX1778 regulates reactive oxygen species (ROS)-mediated cytotoxicity in a p53- and nicotinic acid phosphoribosyltransferase1 (NAPRT1)-dependent manner. J Biol Chem 2012; 287: 22408–22417.
Xiao Y, Elkins K, Durieux JK, Lee L, Oeh J, Yang LX et al. Dependence of tumor cell lines and patient-derived tumors on the NAD salvage pathway renders them sensitive to NAMPT inhibition with GNE-618. Neoplasia 2013; 15: 1151–1160.
Stanton RC . Glucose-6-phosphate dehydrogenase, NADPH, and cell survival. IUBMB Life 2012; 64: 362–369.
Rabinowitz JD, White E . Autophagy and metabolism. Science 2010; 330: 1344–1348.
Park CW, Hong SM, Kim ES, Kwon JH, Kim KT, Nam HG et al. BNIP3 is degraded by ULK1-dependent autophagy via MTORC1 and AMPK. Autophagy 2013; 9: 345–360.
Rousset M, Zweibaum A, Fogh J . Presence of glycogen and growth-related variations in 58 cultured human tumor cell lines of various tissue origins. Cancer Res 1981; 41: 1165–1170.
Favaro E, Bensaad K, Chong MG, Tennant DA, Ferguson DJ, Snell C et al. Glucose utilization via glycogen phosphorylase sustains proliferation and prevents premature senescence in cancer cells. Cell Metab 2012; 16: 751–764.
Leithner K, Hrzenjak A, Trotzmuller M, Moustafa T, Kofeler HC, Wohlkoenig C et al. PCK2 activation mediates an adaptive response to glucose depletion in lung cancer. Oncogene 2015; 34: 1044–1050.
Choi YJ, Choi SE, Ha ES, Kang Y, Han SJ, Kim DJ et al. Extracellular visfatin activates gluconeogenesis in HepG2 cells through the classical PKA/CREB-dependent pathway. Horm Metab Res 2014; 46: 233–239.
Hsu CP, Oka S, Shao D, Hariharan N, Sadoshima J . Nicotinamide phosphoribosyltransferase regulates cell survival through NAD+ synthesis in cardiac myocytes. Circulation research 2009; 105: 481–491.
Houtkooper RH, Pirinen E, Auwerx J . Sirtuins as regulators of metabolism and healthspan. Nat Rev Mol Cell Biol 2012; 13: 225–238.
Wang YP, Zhou LS, Zhao YZ, Wang SW, Chen LL, Liu LX et al. Regulation of G6PD acetylation by KAT9/SIRT2 modulates NADPH homeostasis and cell survival during oxidative stress. EMBO J 2014; 33: 1304–1320.
Chan DA, Giaccia AJ . Harnessing synthetic lethal interactions in anticancer drug discovery. Nat Rev Drug Discov 2011; 10: 351–364.
Bajrami I, Kigozi A, Van Weverwijk A, Brough R, Frankum J, Lord CJ et al. Synthetic lethality of PARP and NAMPT inhibition in triple-negative breast cancer cells. EMBO Mol Med 2012; 4: 1087–1096.
Jeon SM, Chandel NS, Hay N . AMPK regulates NADPH homeostasis to promote tumour cell survival during energy stress. Nature 2012; 485: 661–665.
Santidrian AF, Matsuno-Yagi A, Ritland M, Seo BB, LeBoeuf SE, Gay LJ et al. Mitochondrial complex I activity and NAD+/NADH balance regulate breast cancer progression. J Clin Invest 2013; 123: 1068–1081.
Acknowledgements
This research was supported by the National R&D Program for Cancer Control, Ministry for Health and Welfare, Korea (1320240), by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MEST) (NRF-2014R1A2A2A01002931), by the Next-Generation BioGreen 21 Program, Rural Development Administration, Republic of Korea (PJ01121601) and by BK21 Plus funded by the Ministry of Education, Korea (10Z20130012243). We thank Kwan-Suk Lee at the POSTECH animal facility for assistance with animal experiments. Slide scanning images were obtained using Virtual Microscope in UNIST-Olympus Biomed Imaging Center (UOBC). We are grateful to Jin-Hoe Hur and UNIST-Olympus Biomed Imaging Center (UOBC) for providing the data of slide scanning images.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Hong, S., Park, C., Kim, S. et al. NAMPT suppresses glucose deprivation-induced oxidative stress by increasing NADPH levels in breast cancer. Oncogene 35, 3544–3554 (2016). https://doi.org/10.1038/onc.2015.415
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2015.415
This article is cited by
-
Circular RNA FEACR inhibits ferroptosis and alleviates myocardial ischemia/reperfusion injury by interacting with NAMPT
Journal of Biomedical Science (2023)
-
Targeting the NAD+ salvage pathway suppresses APC mutation-driven colorectal cancer growth and Wnt/β-catenin signaling via increasing Axin level
Cell Communication and Signaling (2020)
-
Inhibition of nicotinamide phosphoribosyltransferase (NAMPT) with OT-82 induces DNA damage, cell death, and suppression of tumor growth in preclinical models of Ewing sarcoma
Oncogenesis (2020)
-
NAD+ metabolism: pathophysiologic mechanisms and therapeutic potential
Signal Transduction and Targeted Therapy (2020)
-
Suppression of nicotinamide phosphoribosyltransferase expression by miR-154 reduces the viability of breast cancer cells and increases their susceptibility to doxorubicin
BMC Cancer (2019)