Abstract
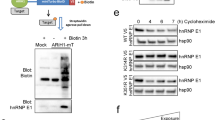
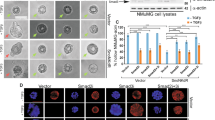
The c-Jun NH2-terminal protein kinase (JNK) pathway has been implicated in mammary tumor development. However, the molecular mechanisms regulating JNK activity in breast cancer cells remain unclear. Here, we report that the inhibition of ubiquitination-like post-translational modification neddylation through different strategies results in enhanced basal JNK phosphorylation in human breast cancer cells. The upregulation of basal JNK phosphorylation upon neddylation inhibition is independent of the deneddylation of Cullins, the well-characterized neddylation substrates. Since augmented basal JNK phosphorylation via ectopic MKK7 expression impedes proliferation and the epithelial-to-mesenchymal transition (EMT) phenotype, the neddylation system might contribute to mammary tumor development partially through limiting basal JNK phosphorylation. Further exploration reveals that MKK7, a JNK-specific MAP2K, undergoes neddylation in human breast cancer cells. MKK7 co-precipitates with a fragment of Ran-binding protein 2 (RanBP2), a large multimodular and pleiotropic protein that has been recognized as a SUMO E3 ligase. Knockdown of RanBP2 attenuates MKK7 neddylation and augments basal JNK phosphorylation without affecting the neddylation of Cullins, whereas ectopic expression of a RanBP2 fragment possessing SUMO E3 activity (RanBP2ΔFG) manifests the opposite effects. In vitro neddylation assays confirm that RanBP2ΔFG works as the neddylation E3 ligase for MKK7. The basal kinase activity of endogenous MKK7 increases upon RanBP2 knockdown but decreases upon the ectopic expression of RanBP2ΔFG. Furthermore, purified MKK7 shows reduced basal kinase activity after in vitro neddylation by RanBP2ΔFG. Consistently, RanBP2 knockdown leads to reduced proliferation and impaired EMT phenotype in human breast cancer cells and the effects of RanBP2 knockdown are reversed by simultaneous MKK7 knockdown. Taken together, our data suggest that MKK7 undergoes neddylation in human breast cancer cells, which limits its basal kinase activity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Hibi M, Lin A, Smeal T, Minden A, Karin M . Identification of an oncoprotein- and UV-responsive protein kinase that binds and potentiates the c-Jun activation domain. Genes Dev 1993; 7: 2135–2148.
Weston CR, Davis RJ . The JNK signal transduction pathway. Curr Opin Cell Biol 2007; 19: 1–8.
Yu C, Minemoto Y, Zhang J, Liu J, Tang F, Bui TN, Xiang J et al. JNK suppresses apoptosis via phosphorylation of the proapoptotic Bcl-2 family protein BAD. Mol Cell 2004; 13: 329–340.
Lin A . Activation of the JNK signaling pathway: breaking the brake on apoptosis. Bioessays 2003; 25: 17–24.
Lu X, Nemoto S, Lin A . Identification of c-Jun NH2-terminal protein kinase (JNK)-activating kinase 2 as an activator of JNK but not p38. J Biol Chem 1997; 272: 24751–24754.
Cui J, Wang Q, Wang J, Lv M, Zhu N, Li Y et al. Basal c-Jun NH2-terminal protein kinase activity is essential for survival and proliferation of T-cell acute lymphoblastic leukemia cells. Mol Cancer Ther 2009; 8: 3214–3222.
Guo Y, Wang W, Wang J, Feng J, Wang Q, Jin J et al. Receptor for activated kinase 1 promotes hepatocellular carcinoma growth by enhancing mitogen-activated protein kinase kinase 7 activity. Hepatology 2013; 57: 140–151.
Wang J, Tang R, Lv M, Wang Q, Zhang X, Guo Y et al. Defective anchoring of JNK1 in the cytoplasm by MKK7 in Jurkat cells is associated with resistance to Fas-mediated apoptosis. Mol Biol Cell 2011; 22: 117–127.
Ellis MJ, Ding L, Shen D, Luo J, Suman VJ, Wallis JW et al. Whole-genome analysis informs breast cancer response to aromatase inhibition. Nature 2012; 486: 353–360.
Curtis C, Shah SP, Chin SF, Turashvili G, Reda OM, Dunning MJ et al. The genomic and transcriptomic architecture of 2,000 breast tumours reveals novel subgroups. Nature 2012; 486: 346–352.
Schramek D, Kotsinas A, Meixner A, Wada T, Elling U, Pospisilik JA et al. The stress kinase MKK7 couples oncogenic stress to p53 stability and tumor suppression. Nat Genet 2011; 43: 212–219.
Cellurale C, Girnius N, Jiang F, Cavanagh-Kyros J, Lu S, Garlick DS et al. Role of JNK in mammary gland development and breast cancer. Cancer Res 2012; 72: 472–481.
Cellurale C, Weston CR, Reilly J, Garlick DS, Jerry DJ, Sluss HK et al. Role of JNK in a Trp53-dependent mouse model of breast cancer. PLoS One 2010; 5: e12469.
Chen P, O’Neal JF, Ebelt ND, Cantrell MA, Mitra S, Nasrazadani A et al. Jnk2 effects on tumor development, genetic instability and replicative stress in an oncogene-driven mouse mammary tumor model. PLoS One 2010; 5: e10443.
Xirodimas DP . Novel substrates and functions for the ubiquitin-like molecule NEDD8. Biochem Soc Trans 2008; 36: 802–806.
Tateishi K, Omata M, Tanaka K, Chiba T . The NEDD8 system is essential for cell cycle progression and morphogenetic pathway in mice. J Cell Biol 2001; 155: 571–579.
Xie P, Zhang M, He S, Lu K, Chen Y, Xing G et al. The covalent modifier Nedd8 is critical for the activation of Smurf1 ubiquitin ligase in tumorigenesis. Nat Commun 2014; 5: 3733.
Kim W, Bennett EJ, Huttlin EL, Guo A, Li J, Possemato A et al. Systematic and quantitative assessment of the ubiquitin-modified proteome. Mol Cell 2011; 44: 325–340.
Swords RT, Kelly KR, Smith PG, Garnsey JJ, Mahalingam D, Medina E et al. Inhibition of NEDD8-activating enzyme: a novel approach for the treatment of acute myeloid leukemia. Blood 2010; 115: 3796–3800.
Godbersen JC, Humphries LA, Danilova OV, Kebbekus PE, Brown JR, Eastman A et al. The Nedd8-activating enzyme inhibitor MLN4924 thwarts microenvironment-driven NF-κB activation and induces apoptosis in chronic lymphocytic leukemia B cells. Clin Cancer Res 2014; 20: 1576–1589.
Hjerpe R, Thomas Y, Chen J, Zemla A, Curran S, Shpiro N et al. Changes in the ratio of free NEDD8 to ubiquitin triggers NEDDylation by ubiquitin enzymes. Biochem J 2012; 441: 927–936.
Pelham CJ, Ketsawatsomkron P, Groh S, Grobe JL, de Lange WJ, Ibeawuchi SRC et al. Cullin-3 regulates vascular smooth muscle function and arterial blood pressure via PPARγ and RhoA/Rho-kinase. Cell Metab 2012; 16: 462–472.
Collier-Hyams LS, Sloane VS, Batten BC, Neish AS . Bacterial modulation of epithelial signaling via changes in neddylation of Cullin-1. J Immunol 2005; 175: 4194–4198.
Jubelin G, Taieb F, Duda DM, Hsu Y, Samba-Louaka A, Nobe R et al. Pathogenic bacteria target NEDD8-conjugated cullins to hijack host-cell signaling pathways. PLoS Pathog 2010; 6: e1001128.
Soucy TA, Smith PG, Milhollen MA, Berger AJ, Gavin JM, Adhikari S et al. An inhibitor of NEDD8-activating enzyme as a new approach to treat cancer. Nature 2009; 458: 732–736.
Kamura T, Conrad MN, Yan Q, Conaway RC, Conaway JW . The Rbx1 subunit of SCF and VHL E3 ubiquitin ligase activates Rub1 modification of cullins Cdc53 and Cul2. Genes Dev 1999; 13: 2928–2933.
Zhang Z, Yang M, Chen R, Su W, Li P, Shen S et al. IBO regulates epithelial-to-mesenchymal transition and the motility of breast cancer cells via Rac1, RhoA and Cdc42 signaling pathways. Oncogene 2014; 33: 3374–3382.
Zeisberg M, Neilson EG . Biomarkers for epithelial-mesenchymal transitions. J Clin Invest 1999; 119: 1429–1437.
Xirodimas DP, Saville MK, Bourdon JC, Hay RT, Lane DP . Mdm2-mediated NEDD8 conjugation of p53 inhibits its transcriptional activity. Cell 2004; 118: 83–97.
Aoki I, Higuchi M, Gotoh Y . NEDDylation controls the target specificity of E2F1 and apoptosis induction. Oncogene 2013; 32: 3954–3964.
Hutten S, Flotho A, Melchior F, Kehlenbach RH . The Nup358-RanGAP complex is required for efficient importin α/β-dependent nuclear import. Mol Biol cell 2008; 19: 2300–2310.
Enchev RI, Schulman BA, Peter M . Protein neddylation: beyond cullin-RING ligases. Nat Rev Mol Cell Biol 2015; 16: 30–44.
Broemer M, Tenev T, Rigbolt KTG, Hempel S, Blagoev B, Silke J et al. Systematic in vivo RNAi analysis identifies IAPs as NEDD8-E3 ligases. Mol Cell 2010; 40: 810–822.
Embade N, Fernandez-Ramos D, Varela-Rey M, Beraza N, Sini M, Gutierrez de Juan V et al. Murine double minute 2 regulates Hu antigen R stabbility in human liver and colon cancer through NEDDylation. Hepatology 2012; 55: 1237–1248.
Wang J, Tang R, Lv M, Wang Q, Zhang X, Guo Y et al. Defective anchoring of JNK1 in the cytoplasm by MKK7 in Jurkat cells is associated with resistance to Fas-mediated apoptosis. Mol Biol Cell 2011; 22: 117–127.
Zhang J, Zhu N, Wang Q, Wang J, Ma Y, Qiao C et al. MEKK3 over-expression contributes to the hyper-responsiveness of IL-12-overproducing cells and CD4+ T conventional cells in NOD mice. J Immunol 2010; 185: 3554–3563.
Zhang J, Wang Q, Zhu N, Yu M, Shen B, Xiang J et al. Cyclic AMP inhibits JNK activation by CREB-mediated induction of c-FLIPL and MKP-1, thereby antagonizing UV-induced apoptosis. Cell Death Differ 2008; 15: 1654–1662.
Acknowledgements
We thank Professors Ming Shi, Ning Guo, Ailing Li and Xuemin Zhang for providing reagents. This work was supported by grants from National Natural Science Foundation of China (81472736, 31270960).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Zhu, T., Wang, J., Pei, Y. et al. Neddylation controls basal MKK7 kinase activity in breast cancer cells. Oncogene 35, 2624–2633 (2016). https://doi.org/10.1038/onc.2015.323
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2015.323