Abstract
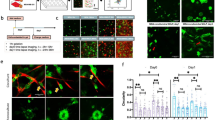
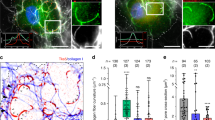
Metastatic invasion of tumors into peripheral tissues is known to rely upon protease-mediated degradation of the surrounding stroma. This remodeling process uses complex, actin-based, specializations of the plasma membrane termed invadopodia that act both to sequester and release matrix metalloproteinases. Here we report that cells of mesenchymal origin, including tumor-associated fibroblasts, degrade substantial amounts of surrounding matrix by a mechanism independent of conventional invadopodia. These degradative sites lack the punctate shape of conventional invadopodia to spread along the cell base and are reticular and/or fibrous in character. In marked contrast to invadopodia, this degradation does not require the action of Src kinase, Cdc42 or Dyn2. Rather, inhibition of Dyn2 causes a marked upregulation of stromal matrix degradation. Further, expression and activity of matrix metalloproteinases are differentially regulated between tumor cells and stromal fibroblasts. This matrix remodeling by fibroblasts increases the invasive capacity of tumor cells, thereby illustrating how the tumor microenvironment can contribute to metastasis. These findings provide evidence for a novel matrix remodeling process conducted by stromal fibroblasts that is substantially more effective than conventional invadopodia, distinct in structural organization and regulated by disparate molecular mechanisms.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Murphy DA, Courtneidge SA . The 'ins' and 'outs' of podosomes and invadopodia: characteristics, formation and function. Nat Rev Mol Cell Biol 2011; 12: 413–426.
Baldassarre M, Pompeo A, Beznoussenko G, Castaldi C, Cortellino S, McNiven MA et al. Dynamin participates in focal extracellular matrix degradation by invasive cells. Mol Biol Cell 2003; 14: 1074–1084.
Yamaguchi H, Lorenz M, Kempiak S, Sarmiento C, Coniglio S, Symons M et al. Molecular mechanisms of invadopodium formation: the role of the N-WASP-Arp2/3 complex pathway and cofilin. J Cell Biol 2005; 168: 441–452.
Chen WT, Chen JM, Parsons SJ, Parsons JT . Local degradation of fibronectin at sites of expression of the transforming gene product pp60src. Nature 1985; 316: 156–158.
Buccione R, Orth JD, McNiven MA . Foot and mouth: podosomes, invadopodia and circular dorsal ruffles. Nat Rev Mol Cell Biol 2004; 5: 647–657.
Waghray M, Yalamanchili M, di Magliano MP, Simeone DM . Deciphering the role of stroma in pancreatic cancer. Curr Opin Gastroenterol 2013; 29: 537–543.
Gaggioli C, Hooper S, Hidalgo-Carcedo C, Grosse R, Marshall JF, Harrington K et al. Fibroblast-led collective invasion of carcinoma cells with differing roles for RhoGTPases in leading and following cells. Nat Cell Biol 2007; 9: 1392–1400.
Brentnall TA, Lai LA, Coleman J, Bronner MP, Pan S, Chen R . Arousal of cancer-associated stroma: overexpression of palladin activates fibroblasts to promote tumor invasion. PLoS One 2012; 7: e30219.
Goicoechea SM, Garcia-Mata R, Staub J, Valdivia A, Sharek L, McCulloch CG et al. Palladin promotes invasion of pancreatic cancer cells by enhancing invadopodia formation in cancer-associated fibroblasts. Oncogene 2014; 33: 1265–1273.
Destaing O, Ferguson SM, Grichine A, Oddou C, De Camilli P, Albiges-Rizo C et al. Essential function of dynamin in the invasive properties and actin architecture of v-Src induced podosomes/invadosomes. PLoS One 2013; 8: e77956.
Artym VV, Zhang Y, Seillier-Moiseiwitsch F, Yamada KM, Mueller SC . Dynamic interactions of cortactin and membrane type 1 matrix metalloproteinase at invadopodia: defining the stages of invadopodia formation and function. Cancer Res 2006; 66: 3034–3043.
Ohlund D, Elyada E, Tuveson D . Fibroblast heterogeneity in the cancer wound. J Exp Med 2014; 211: 1503–1523.
Wang Y, McNiven MA . Invasive matrix degradation at focal adhesions occurs via protease recruitment by a FAK-p130Cas complex. J Cell Biol 2012; 196: 375–385.
Yu X, Zech T, McDonald L, Gonzalez EG, Li A, Macpherson I et al. N-WASP coordinates the delivery and F-actin-mediated capture of MT1-MMP at invasive pseudopods. J Cell Biol 2012; 199: 527–544.
Ferguson SM, Raimondi A, Paradise S, Shen H, Mesaki K, Ferguson A et al. Coordinated actions of actin and BAR proteins upstream of dynamin at endocytic clathrin-coated pits. Dev Cell 2009; 17: 811–822.
Boateng LR, Huttenlocher A . Spatiotemporal regulation of Src and its substrates at invadosomes. Eur J Cell Biol 2012; 91: 878–888.
Nakahara H, Otani T, Sasaki T, Miura Y, Takai Y, Kogo M . Involvement of Cdc42 and Rac small G proteins in invadopodia formation of RPMI7951 cells. Genes Cells 2003; 8: 1019–1027.
Razidlo GL, Schroeder B, Chen J, Billadeau DD, McNiven MA . Vav1 as a central regulator of invadopodia assembly. Curr Biol 2014; 24: 86–93.
Sato H, Takino T, Kinoshita T, Imai K, Okada Y, Stetler Stevenson WG et al. Cell surface binding and activation of gelatinase A induced by expression of membrane-type-1-matrix metalloproteinase (MT1-MMP). FEBS Lett 1996; 385: 238–240.
Okada A, Tomasetto C, Lutz Y, Bellocq JP, Rio MC, Basset P . Expression of matrix metalloproteinases during rat skin wound healing: evidence that membrane type-1 matrix metalloproteinase is a stromal activator of pro-gelatinase A. J Cell Biol 1997; 137: 67–77.
Jiang A, Lehti K, Wang X, Weiss SJ, Keski-Oja J, Pei D . Regulation of membrane-type matrix metalloproteinase 1 activity by dynamin-mediated endocytosis. Proc Natl Acad Sci USA 2001; 98: 13693–13698.
Rhim AD, Oberstein PE, Thomas DH, Mirek ET, Palermo CF, Sastra SA et al. Stromal elements act to restrain, rather than support, pancreatic ductal adenocarcinoma. Cancer Cell 2014; 25: 735–747.
Ozdemir BC, Pentcheva-Hoang T, Carstens JL, Zheng X, Wu CC, Simpson TR et al. Depletion of carcinoma-associated fibroblasts and fibrosis induces immunosuppression and accelerates pancreas cancer with reduced survival. Cancer Cell 2014; 25: 719–734.
Martinez-Outschoorn UE, Lisanti MP, Sotgia F . Catabolic cancer-associated fibroblasts transfer energy and biomass to anabolic cancer cells, fueling tumor growth. Semin Cancer Biol 2014; 25: 47–60.
Egeblad M, Littlepage LE, Werb Z . The fibroblastic coconspirator in cancer progression. Cold Spring Harbor Symposia on Quantitative Biology 2005; 70: 383–388.
Calon A, Tauriello DV, Batlle E . TGF-beta in CAF-mediated tumor growth and metastasis. Semin Cancer Biol 2014; 25: 15–22.
Mishra P, Banerjee D, Ben-Baruch A . Chemokines at the crossroads of tumor-fibroblast interactions that promote malignancy. J Leukoc Biol 2011; 89: 31–39.
Luga V, Wrana JL . Tumor-stroma interaction: revealing fibroblast-secreted exosomes as potent regulators of Wnt-planar cell polarity signaling in cancer metastasis. Cancer Res 2013; 73: 6843–6847.
De Wever O, Van Bockstal M, Mareel M, Hendrix A, Bracke M . Carcinoma-associated fibroblasts provide operational flexibility in metastasis. Semin Cancer Biol 2014; 25: 33–46.
Karagiannis GS, Poutahidis T, Erdman SE, Kirsch R, Riddell RH, Diamandis EP . Cancer-associated fibroblasts drive the progression of metastasis through both paracrine and mechanical pressure on cancer tissue. Mol Cancer Res 2012; 10: 1403–1418.
Cao H, Orth JD, Chen J, Weller SG, Heuser JE, McNiven MA . Cortactin is a component of clathrin-coated pits and participates in receptor-mediated endocytosis. Mol Cell Biol 2003; 23: 2162–2170.
Cao H, Garcia F, McNiven MA . Differential distribution of dynamin isoforms in mammalian cells. Mol Biol Cell 1998; 9: 2595–2609.
Cao H, Thompson HM, Krueger EW, McNiven MA . Disruption of Golgi structure and function in mammalian cells expressing a mutant dynamin. J Cell Sci 2000; 113: 1993–2002.
McNiven MA, Kim L, Krueger EW, Orth JD, Cao H, Wong TW . Regulated interactions between dynamin and the actin-binding protein cortactin modulate cell shape. J Cell Biol 2000; 151: 187–198.
Eppinga RD, Krueger EW, Weller SG, Zhang L, Cao H, McNiven MA . Increased expression of the large GTPase dynamin 2 potentiates metastatic migration and invasion of pancreatic ductal carcinoma. Oncogene 2012; 31: 1228–1241.
Ries C, Egea V, Karow M, Kolb H, Jochum M, Neth P . MMP-2, MT1-MMP, and TIMP-2 are essential for the invasive capacity of human mesenchymal stem cells: differential regulation by inflammatory cytokines. Blood 2007; 109: 4055–4063.
Henley JR, Krueger EW, Oswald BJ, McNiven MA . Dynamin-mediated internalization of caveolae. J Cell Biol 1998; 141: 85–99.
Acknowledgements
This work was supported by R01 CA104125 to MAM, P30DK084567 (Mayo Clinic Center for Cell Signaling in Gastroenterology), T32 DK007198 (to RDE), and P50 CA102701 (Mayo Clinic SPORE in Pancreatic Cancer, to GLR).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Rights and permissions
About this article
Cite this article
Cao, H., Eppinga, R., Razidlo, G. et al. Stromal fibroblasts facilitate cancer cell invasion by a novel invadopodia-independent matrix degradation process. Oncogene 35, 1099–1110 (2016). https://doi.org/10.1038/onc.2015.163
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2015.163
This article is cited by
-
Development of a model for fibroblast-led collective migration from breast cancer cell spheroids to study radiation effects on invasiveness
Radiation Oncology (2021)
-
Metastasis-associated fibroblasts: an emerging target for metastatic cancer
Biomarker Research (2021)
-
Cancer-associated fibroblasts promote the progression of endometrial cancer via the SDF-1/CXCR4 axis
Journal of Hematology & Oncology (2016)
-
Actomyosin-dependent dynamic spatial patterns of cytoskeletal components drive mesoscale podosome organization
Nature Communications (2016)