Abstract
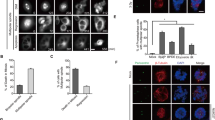
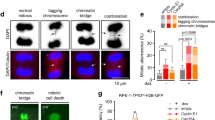
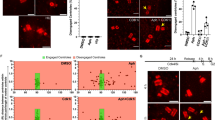
The antineoplastic drug hydroxyurea (HU), when used at subtoxic doses, induces prolonged replication stress and centrosome amplification. This causes genomic instability and increases the malignancy of the recurring tumor. The mechanism of centrosome amplification induced by prolonged replication stress, however, is still unclear. Here, we examined the involvement of ataxia telangiectasia, mutated (ATM), ataxia telangiectasia, mutated and Rad3-related (ATR) and DNA-dependent protein kinase (DNA-PK) and found that HU-induced centrosome amplification was inhibited by the depletion of DNA-PKcs, but not ATM and ATR. Inactivation of ATM/ATR in U2OS cells instead caused aneuploidy and cell death. We found DNA-PKcs depletion also abrogated ATM phosphorylation, indicating that ATM activation during prolonged replication stress depends on DNA-PK. Depletion of DNA-PK abrogated checkpoint kinase (Chk)2 activation and partially reduced Chk1 activation. Chk2 depletion blocked HU-induced centrosome amplification, indicating a function of Chk2 in centrosome amplification. We further found that Chk2 was phosphorylated at Thr68 on the mother centriole at late G2 and mitosis when unstressed and on all amplified centrioles induced by HU. In summary, we have elucidated that DNA-PK/Chk2 signaling induces centrosome amplification upon long-term HU treatment, therefore increasing our insight into tumor recurrence after initial chemotherapy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Stehman FB, Bundy BN, Kucera PR, Deppe G, Reddy S, O'Connor DM . Hydroxyurea 5-fluorouracil infusion, and cisplatin adjunct to radiation therapy in cervical carcinoma: a phase I-II trial of the Gynecologic Oncology Group. Gynecol Oncol 1997; 66: 262–267.
Prosser SL, Straatman KR, Fry AM . Molecular dissection of the centrosome overduplication pathway in S-phase-arrested cells. Mol Cell Biol 2009; 29: 1760–1773.
Khodjakov A, Rieder CL, Sluder G, Cassels G, Sibon O, Wang CL . De novo formation of centrosomes in vertebrate cells arrested during S phase. J Cell Biol 2002; 158: 1171–1181.
Sato N, Mizumoto K, Nakamura M, Ueno H, Minamishima YA, Farber JL et al. A possible role for centrosome overduplication in radiation-induced cell death. Oncogene 2000; 19: 5281–5290.
Bennett RA, Izumi H, Fukasawa K . Induction of centrosome amplification and chromosome instability in p53-null cells by transient exposure to subtoxic levels of S-phase-targeting anticancer drugs. Oncogene 2004; 23: 6823–6829.
Durocher D, Jackson SP . DNA-PK, ATM and ATR as sensors of DNA damage: variations on a theme? Curr Opin Cell Biol 2001; 13: 225–231.
Shiloh Y . ATM and related protein kinases: safeguarding genome integrity. Nat Rev Cancer 2003; 3: 155–168.
Bakkenist CJ, Kastan MB . DNA damage activates ATM through intermolecular autophosphorylation and dimer dissociation. Nature 2003; 421: 499–506.
Stiff T, Walker SA, Cerosaletti K, Goodarzi AA, Petermann E, Concannon P et al. ATR-dependent phosphorylation and activation of ATM in response to UV treatment or replication fork stalling. EMBO J 2006; 25: 5775–5782.
Smith J, Tho LM, Xu N, Gillespie DA . The ATM-Chk2 and ATR-Chk1 pathways in DNA damage signaling and cancer. Adv Cancer Res 2010; 108: 73–112.
Dodson H, Bourke E, Jeffers LJ, Vagnarelli P, Sonoda E, Takeda S et al. Centrosome amplification induced by DNA damage occurs during a prolonged G2 phase and involves ATM. EMBO J 2004; 23: 3864–3873.
Bozulic L, Surucu B, Hynx D, Hemmings BA . PKBalpha/Akt1 acts downstream of DNA-PK in the DNA double-strand break response and promotes survival. Mol Cell 2008; 30: 203–213.
Collis SJ, DeWeese TL, Jeggo PA, Parker AR . The life and death of DNA-PK. Oncogene 2005; 24: 949–961.
Zou L, Elledge SJ . Sensing DNA damage through ATRIP recognition of RPA-ssDNA complexes. Science 2003; 300: 1542–1548.
Bourke E, Brown JA, Takeda S, Hochegger H, Morrison CG . DNA damage induces Chk1-dependent threonine-160 phosphorylation and activation of Cdk2. Oncogene 2010; 29: 616–624.
Robinson HM, Black EJ, Brown R, Gillespie DA . DNA mismatch repair and Chk1-dependent centrosome amplification in response to DNA alkylation damage. Cell Cycle 2007; 6: 982–992.
Wang CY, Kao YH, Lai PY, Chen WY, Chung BC . Steroidogenic factor-1 (NR5A1) maintains centrosome homeostasis in steroidogenic cells by restricting centrosomal DNA-PK activation. Mol Cell Biol 2013; 33: 476–484.
Liu Q, Guntuku S, Cui XS, Matsuoka S, Cortez D, Tamai K et al. Chk1 is an essential kinase that is regulated by Atr and required for the G(2)/M DNA damage checkpoint. Genes Dev 2000; 14: 1448–1459.
Nakagawa Y, Yamane Y, Okanoue T, Tsukita S, Tsukita S . Outer dense fiber 2 is a widespread centrosome scaffold component preferentially associated with mother centrioles: its identification from isolated centrosomes. Mol Biol Cell 2001; 12: 1687–1697.
Lee KJ, Lin YF, Chou HY, Yajima H, Fattah KR, Lee SC et al. Involvement of DNA-dependent protein kinase in normal cell cycle progression through mitosis. J Biol Chem 2011; 286: 12796–12802.
Stolz A, Ertych N, Kienitz A, Vogel C, Schneider V, Fritz B et al. The CHK2-BRCA1 tumour suppressor pathway ensures chromosomal stability in human somatic cells. Nat Cell Biol 2010; 12: 492–499.
Shang ZF, Huang B, Xu QZ, Zhang SM, Fan R, Liu XD et al. Inactivation of DNA-dependent protein kinase leads to spindle disruption and mitotic catastrophe with attenuated checkpoint protein 2 Phosphorylation in response to DNA damage. Cancer Res 2010; 70: 3657–3666.
Golan A, Pick E, Tsvetkov L, Nadler Y, Kluger H, Stern DF . Centrosomal Chk2 in DNA damage responses and cell cycle progression. Cell Cycle 2010; 9: 2647–2656.
Tsvetkov L, Xu X, Li J, Stern DF . Polo-like kinase 1 and Chk2 interact and co-localize to centrosomes and the midbody. J Biol Chem 2003; 278: 8468–8475.
Joo K, Kim CG, Lee MS, Moon HY, Lee SH, Kim MJ et al. CCDC41 is required for ciliary vesicle docking to the mother centriole. Proc Natl Acad Sci USA 2013; 110: 5987–5992.
Graser S, Stierhof YD, Lavoie SB, Gassner OS, Lamla S, Le Clech M et al. Cep164, a novel centriole appendage protein required for primary cilium formation. J Cell Biol 2007; 179: 321–330.
An J, Yang DY, Xu QZ, Zhang SM, Huo YY, Shang ZF et al. DNA-dependent protein kinase catalytic subunit modulates the stability of c-Myc oncoprotein. Mol Cancer 2008; 7: 32.
Ayene IS, Ford LP, Koch CJ . Ku protein targeting by Ku70 small interfering RNA enhances human cancer cell response to topoisomerase II inhibitor and gamma radiation. Mol Cancer Ther 2005; 4: 529–536.
Rampakakis E, Di Paola D, Zannis-Hadjopoulos M . Ku is involved in cell growth DNA replication and G1-S transition. J Cell Sci 2008; 121: 590–600.
Andreassen PR, D'Andrea AD, Taniguchi T . ATR couples FANCD2 monoubiquitination to the DNA-damage response. Genes Dev 2004; 18: 1958–1963.
Kramer A, Mailand N, Lukas C, Syljuasen RG, Wilkinson CJ, Nigg EA et al. Centrosome-associated Chk1 prevents premature activation of cyclin-B-Cdk1 kinase. Nat Cell Biol 2004; 6: 884–891.
Acknowledgements
We would like to thank Ya-Min Lin for technical assistance in FACS analysis. This study was supported by grants from Academia Sinica, NHRI-EX102-10210SI and NSC102-2923-B-001-003-MY3 to B-cC and NSC102-2320-B-006–051 to C-YW.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Wang, CY., Huang, EH., Huang, Sc. et al. DNA-PK/Chk2 induces centrosome amplification during prolonged replication stress. Oncogene 34, 1263–1269 (2015). https://doi.org/10.1038/onc.2014.74
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2014.74
This article is cited by
-
CHK2 activation contributes to the development of oxaliplatin resistance in colorectal cancer
British Journal of Cancer (2022)
-
Centrosome, microtubule and DNA damage response
Genome Instability & Disease (2022)
-
Genotoxic stress-activated DNA-PK-p53 cascade and autophagy cooperatively induce ciliogenesis to maintain the DNA damage response
Cell Death & Differentiation (2021)
-
Centrosomes in the DNA damage response—the hub outside the centre
Chromosome Research (2016)
-
Chloroquine alleviates etoposide-induced centrosome amplification by inhibiting CDK2 in adrenocortical tumor cells
Oncogenesis (2015)