Abstract
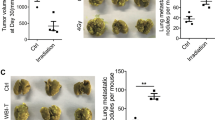
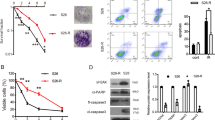
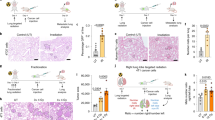
Despite ionizing radiation (IR) is being widely used as a standard treatment for lung cancer, many evidences suggest that IR paradoxically promotes cancer malignancy. However, its molecular mechanisms underlying radiation-induced cancer progression remain obscure. Here, we report that exposure to fractionated radiation (2 Gy per day for 3 days) induces the secretion of granulocyte-colony-stimulating factor (G-CSF) that has been commonly used in cancer therapies to ameliorate neutropenia. Intriguingly, radiation-induced G-CSF promoted the migratory and invasive properties by triggering the epithelial–mesenchymal cell transition (EMT) in non-small-cell lung cancer cells (NSCLCs). By irradiation, G-CSF was upregulated transcriptionally by β-catenin/TCF4 complex that binds to the promoter region of G-CSF as a transcription factor. Importantly, irradiation increased the stability of β-catenin through the activation of PI3K/AKT (phosphatidylinositol 3-kinase/AKT), thereby upregulating the expression of G-CSF. Radiation-induced G-CSF is recognized by G-CSFR and transduced its intracellular signaling JAK/STAT3 (Janus kinase/signal transducers and activators of transcription), thereby triggering EMT program in NSCLCs. Taken together, our findings suggest that the application of G-CSF in cancer therapies to ameliorate neutropenia should be reconsidered owing to its effect on cancer progression, and G-CSF could be a novel therapeutic target to mitigate the harmful effect of radiotherapy for the treatment of NSCLC.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Abbreviations
- G-CSF:
-
granulocyte colony-stimulating factor
- IR:
-
ionizing radiation
- NSCLC:
-
non-small-cell lung cancer
- EMT:
-
epithelial–mesenchymal cell transition
- siRNA:
-
small interfering RNA.
References
Bernier J, Hall EJ, Giaccia A . Radiation oncology: a century of achievements. Nat Rev Cancer 2004; 4: 737–747.
Ross GM . Induction of cell death by radiotherapy. Endocr Relat Cancer 1999; 6: 41–44.
Dirks PB . Brain tumor stem cells: bringing order to the chaos of brain cancer. J Clin Oncol 2008; 26: 2916–2924.
Squatrito M, Brennan CW, Helmy K, Huse JT, Petrini JH, Holland EC . Loss of ATM/Chk2/p53 pathway components accelerates tumor development and contributes to radiation resistance in gliomas. Cancer Cell 2010; 18: 619–629.
Acosta JC, Gil J . Senescence: a new weapon for cancer therapy. Trends Cell Biol 2012; 22: 211–219.
Burgess AW, Metcalf D . The nature and action of granulocyte–macrophage colony stimulating factors. Blood 1980; 56: 947–958.
Metcalf D . The granulocyte–macrophage colony-stimulating factors. Science 1985; 229: 16–22.
Demetri GD, Griffin JD . Granulocyte colony-stimulating factor and its receptor. Blood 1991; 78: 2791–2808.
Dale DC, Liles WC, Summer WR, Nelson S . Review: granulocyte colony-stimulating factor—role and relationships in infectious diseases. J Infect Dis 1995; 172: 1061–1075.
Diederich K, Sevimli S, Dorr H, Kosters E, Hoppen M, Lewejohann L et al. The role of granulocyte-colony stimulating factor (G-CSF) in the healthy brain: a characterization of G-CSF-deficient mice. J Neurosci 2009; 29: 11572–11581.
Teramachi M, Miyamoto N, Yamamoto Y, Sasaka T, Nakamura T, Kitamura F . A case of large cell carcinoma of the lung which is suspected of producing granulocyte colony-stimulating factor. Nihon Kyobu Shikkan Gakkai zasshi 1992; 30: 1327–1332.
Watanabe M, Ono K, Ozeki Y, Tanaka S, Aida S, Okuno Y . Production of granulocyte-macrophage colony-stimulating factor in a patient with metastatic chest wall large cell carcinoma. Jpn J Clin Oncol 1998; 28: 559–562.
Pylayeva-Gupta Y, Lee KE, Hajdu CH, Miller G, Bar-Sagi D . Oncogenic Kras-induced GM-CSF production promotes the development of pancreatic neoplasia. Cancer Cell 2012; 21: 836–847.
Kowanetz M, Wu X, Lee J, Tan M, Hagenbeek T, Qu X et al, Granulocyte-colony stimulating factor promotes lung metastasis through mobilization of Ly6G+Ly6C+ granulocytes. Proc Natl Acad Sci USA 2010; 107: 21248–21255.
Uemura Y, Kobayashi M, Nakata H, Kubota T, Saito T, Bandobashi K et al. Effects of granulocyte colony-stimulating factor and granulocyte–macrophage colony-stimulating factor on lung cancer: roles of cyclooxygenase-2. Oncol Rep 2007; 17: 955–961.
Young MR, Charboneau S, Lozano Y, Djordjevic A, Young ME . Activation of the protein kinase a signal transduction pathway by granulocyte–macrophage colony-stimulating factor or by genetic manipulation reduces cytoskeletal organization in Lewis lung carcinoma variants. Int J Cancer 1994; 56: 446–451.
Uemura Y, Kobayashi M, Nakata H, Harada R, Kubota T, Taguchi H . Effect of serum deprivation on constitutive production of granulocyte-colony stimulating factor and granulocyte macrophage-colony stimulating factor in lung cancer cells. Int J Cancer 2004; 109: 826–832.
Mroczko B, Szmitkowski M . Hematopoietic cytokines as tumor markers. Clin Chem Lab Med: CCLM / FESCC 2004; 42: 1347–1354.
Mroczko B, Szmitkowski M, Czygier M . Granulocyte colony stimulating factor (G-CSF) in diagnosis and monitoring of non-small-cell lung cancer (NSCLC). Pol Arch Med Wewn 2000; 103: 163–168.
Marino VJ, Roguin LP . The granulocyte colony stimulating factor (G-CSF) activates Jak/STAT and MAPK pathways in a trophoblastic cell line. J Cell Biochem 2008; 103: 1512–1523.
Novak U, Ward AC, Hertzog PJ, Hamilton JA, Paradiso L . Aberrant activation of JAK/STAT pathway components in response to G-CSF, interferon-alpha/beta and interferon-gamma in NFS-60 cells. Growth factors 1996; 13: 251–260.
Huber MA, Azoitei N, Baumann B, Grunert S, Sommer A, Pehamberger H et al. NF-kappaB is essential for epithelial–mesenchymal transition and metastasis in a model of breast cancer progression. J Clin Invest 2004; 114: 569–581.
Wang Z, Li Y, Kong D, Sarkar FH . The role of Notch signaling pathway in epithelial–mesenchymal transition (EMT) during development and tumor aggressiveness. Curr Drug Targets 2010; 11: 745–751.
Jiang YG, Luo Y, He DL, Li X, Zhang LL, Peng T et al. Role of Wnt/beta-catenin signaling pathway in epithelial–mesenchymal transition of human prostate cancer induced by hypoxia-inducible factor-1alpha. Int J Urol 2007; 14: 1034–1039.
Sanchez-Tillo E, de Barrios O, Siles L, Cuatrecasas M, Castells A, Postigo A . Beta-catenin/TCF4 complex induces the epithelial-to-mesenchymal transition (EMT)-activator ZEB1 to regulate tumor invasiveness. Proc Natl Acad Sci USA 2011; 108: 19204–19209.
Prise KM, O'Sullivan JM . Radiation-induced bystander signalling in cancer therapy. Nat Rev Cancer 2009; 9: 351–360.
Multhoff G, Radons J . Radiation, inflammation, and immune responses in cancer. Front Oncol 2012; 2: 58.
Kawamoto A, Yokoe T, Tanaka K, Saigusa S, Toiyama Y, Yasuda H et al. Radiation induces epithelial–mesenchymal transition in colorectal cancer cells. Oncol Rep 2012; 27: 51–57.
Yan S, Wang Y, Yang Q, Li X, Kong X, Zhang N et al. Low-dose radiation-induced epithelial–mesenchymal transition through NF-kappaB in cervical cancer cells. Int J Oncol 2013; 42: 1801–1806.
Liu W, Huang YJ, Liu C, Yang YY, Liu H, Cui JG et al. Inhibition of TBK1 attenuates radiation-induced epithelial–mesenchymal transition of A549 human lung cancer cells via activation of GSK-3beta and repression of ZEB1. Lab Invest 2014; 94: 362–370.
Park BK, Zhang H, Zeng Q, Dai J, Keller ET, Giordano T et al. NF-kappaB in breast cancer cells promotes osteolytic bone metastasis by inducing osteoclastogenesis via GM-CSF. Nat Med 2007; 13: 62–69.
Uemura Y, Kobayashi M, Nakata H, Kubota T, Bandobashi K, Saito T et al. Effects of GM-CSF and M-CSF on tumor progression of lung cancer: roles of MEK1/ERK and AKT/PKB pathways. Int J Mol Med 2006; 18: 365–373.
Su S, Liu Q, Chen J, Chen J, Chen F, He C et al. A positive feedback loop between mesenchymal-like cancer cells and macrophages is essential to breast cancer metastasis. Cancer Cell 2014; 25: 605–620.
Looyenga BD, Hutchings D, Cherni I, Kingsley C, Weiss GJ, Mackeigan JP . STAT3 is activated by JAK2 independent of key oncogenic driver mutations in non-small cell lung carcinoma. PLoS One 2012; 7: e30820.
Jiang R, Jin Z, Liu Z, Sun L, Wang L, Li K . Correlation of activated STAT3 expression with clinicopathologic features in lung adenocarcinoma and squamous cell carcinoma. Mol Diagn Ther 2011; 15: 347–352.
Liu RY, Zeng Y, Lei Z, Wang L, Yang H, Liu Z et al. JAK/STAT3 signaling is required for TGF-beta-induced epithelial–mesenchymal transition in lung cancer cells. Int J Oncol 2014; 44: 1643–1651.
Wendt MK, Balanis N, Carlin CR, Schiemann WP . STAT3 and epithelial–mesenchymal transitions in carcinomas. Jak-Stat 2014; 3: e28975.
Rokavec M, Oner MG, Li H, Jackstadt R, Jiang L, Lodygin D et al. IL-6 R/STAT3/miR-34a feedback loop promotes EMT-mediated colorectal cancer invasion and metastasis. J Clin Invest 2014; 124: 1853–1867.
Clevers H, Nusse R . Wnt/beta-catenin signaling and disease. Cell 2012; 149: 1192–1205.
Fang D, Hawke D, Zheng Y, Xia Y, Meisenhelder J, Nika H et al. Phosphorylation of beta-catenin by AKT promotes beta-catenin transcriptional activity. J Biol Chem 2007; 282: 11221–11229.
Wang WC, Tsai JJ, Kuo CY, Chen HM, Kao SH . Non-proteolytic house dust mite allergen, Der p 2, upregulated expression of tight junction molecule claudin-2 associated with Akt/GSK-3beta/beta-catenin signaling pathway. J Cell Biochem 2011; 112: 1544–1551.
Zhang J, Shemezis JR, McQuinn ER, Wang J, Sverdlov M, Chenn A . AKT activation by N-cadherin regulates beta-catenin signaling and neuronal differentiation during cortical development. Neural Dev 2013; 8: 7.
Acknowledgements
This work was supported by the National Research Foundation (NRF) and Ministry of Science, ICT and Future Planning, Korean Government, through its National Nuclear Technology Program (2012M2A2A7035878), and the Ministry of Trade, Industry and Energy (Grant No: 20131610101840).
Author Contributions
Y-HC: conception and design, collection and assembly of data, data analysis and interpretation; YS: conception and design, data analysis and interpretation, manuscript writing; H-JL: data analysis and interpretation; K-CY: data analysis and interpretation; NU: data analysis and interpretation; Y-JJ: data analysis and interpretation; J-SL: data analysis and interpretation; S-GH: data analysis and interpretation; S-YN: data analysis and interpretation; M-JK: conception and design, collection and assembly of data, data analysis and interpretation; S-JL: conception and design, data analysis and interpretation, financial support, final approval of manuscript.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Rights and permissions
About this article
Cite this article
Cui, YH., Suh, Y., Lee, HJ. et al. Radiation promotes invasiveness of non-small-cell lung cancer cells through granulocyte-colony-stimulating factor. Oncogene 34, 5372–5382 (2015). https://doi.org/10.1038/onc.2014.466
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2014.466
This article is cited by
-
Tumor-associated macrophages as a potential therapeutic target in thyroid cancers
Cancer Immunology, Immunotherapy (2023)
-
YM155 decreases radiation-induced invasion and reverses epithelial–mesenchymal transition by targeting STAT3 in glioblastoma
Journal of Translational Medicine (2018)
-
Effects of radiation on the metastatic process
Molecular Medicine (2018)
-
FBXL14 abolishes breast cancer progression by targeting CDCP1 for proteasomal degradation
Oncogene (2018)
-
Induction of metastasis, cancer stem cell phenotype, and oncogenic metabolism in cancer cells by ionizing radiation
Molecular Cancer (2017)