Abstract
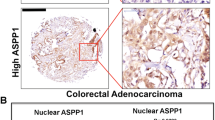
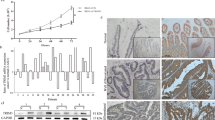
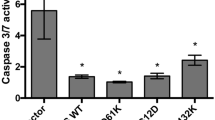
The myristoylated alanine-rich C-kinase substrate (MARCKS) acts as a tumor suppressor in a variety of human neoplasms. In colorectal cancers (CRCs), MARCKS has been shown to be a preferential target of mutational inactivation in tumors following the microsatellite instability (MSI-H) pathway but little is known about its impact on intestinal carcinogenesis. To investigate the relevance of MARCKS inactivation in more detail, we analyzed 926 MSI-typed CRCs for MARCKS expression by immunohistochemistry and studied the functional consequences of MARCKS depletion in colorectal cancer cell lines. We found that loss of MARCKS expression was not restricted to MSI-H cancers but also occurred in microsatellite stable (MSS) tumors, where it was associated with an adverse outcome regarding overall survival, cancer-specific and disease-free survival (P=0.002, P=0.0018, P=0.0001, respectively; univariate analysis). In MARCKS-positive MSS colon cancer cell lines (SW480 and SW707) small interfering RNA (siRNA)-mediated knockdown of MARCKS conferred resistance to tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-induced apoptosis. This was accompanied by the downregulation of the TRAIL receptors DR4 and DR5 at the cell surface and activation of AKT signaling. Inhibition of AKT signaling and transient overexpression of wild-type MARCKS, but not of MARCKS lacking the effector domain (ED), abolished the anti-apoptotic effect. In conclusion, our data show that inactivation of MARCKS is common in CRCs and is associated with adverse outcome in MSS cancers. The finding that MARCKS acts as a mediator of apoptosis in MSS CRC cells adds a novel tumor-suppressing function to the so far established roles of MARCKS in cell motility and proliferation and can explain the prognostic effect of MARCKS depletion in MSS CRC.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Albert KA, Nairn AC, Greengard P . The 87-kDa protein, a major specific substrate for protein kinase C: purification from bovine brain and characterization. Proc Natl Acad Sci USA 1987; 84: 7046–7050.
Stumpo DJ, Graff JM, Albert KA, Greengard P, Blackshear PJ . Molecular cloning, characterization, and expression of a cDNA encoding the ‘80- to 87-kDa’ myristoylated alanine-rich C kinase substrate: a major cellular substrate for protein kinase C. Proc Natl Acad Sci USA 1989; 86: 4012–4016.
Seykora JT, Ravetch JV, Aderem A . Cloning and molecular characterization of the murine macrophage ‘68-kDa’ protein kinase C substrate and its regulation by bacterial lipopolysaccharide. Proc Natl Acad Sci USA 1991; 88: 2505–2509.
Harlan DM, Graff JM, Stumpo DJ, Eddy RL Jr, Shows TB, Boyle JM et al The human myristoylated alanine-rich C kinase substrate (MARCKS) gene (MACS). Analysis of its gene product, promoter, and chromosomal localization. J Biol Chem 1991; 266: 14399–14405.
Graff JM, Young TN, Johnson JD, Blackshear PJ . Phosphorylation-regulated calmodulin binding to a prominent cellular substrate for protein kinase C. J Biol Chem 1989; 264: 21818–21823.
Arbuzova A, Schmitz AA, Vergeres G . Cross-talk unfolded: MARCKS proteins. Biochem J 2002; 362: 1–12.
Laux T, Fukami K, Thelen M, Golub T, Frey D, Caroni P . GAP43, MARCKS, and CAP23 modulate PI(4,5)P(2) at plasmalemmal rafts, and regulate cell cortex actin dynamics through a common mechanism. J Cell Biol 2000; 149: 1455–1472.
Brooks G, Brooks SF, Goss MW . MARCKS functions as a novel growth suppressor in cells of melanocyte origin. Carcinogenesis 1996; 17: 683–689.
Rose SD, Cook HW, Palmer FB, Ridgway ND, Byers DM . Differential expression of MARCKS and other calmodulin-binding protein kinase C substrates in cultured neuroblastoma and glioma cells. J Neurochem 1994; 63: 2314–2323.
Joseph CK, Qureshi SA, Wallace DJ, Foster DA . MARCKS protein is transcriptionally down-regulated in v-Src-transformed BALB/c 3T3 cells. J Biol Chem 1992; 267: 1327–1330.
Wolfman A, Wingrove TG, Blackshear PJ, Macara IG . Down-regulation of protein kinase C and of an endogenous 80-kDa substrate in transformed fibroblasts. J Biol Chem 1987; 262: 16546–16552.
Simek SL, Kligman D, Patel J, Colburn NH . Differential expression of an 80-kDa protein kinase C substrate in preneoplastic and neoplastic mouse JB6 cells. Proc Natl Acad Sci USA 1989; 86: 7410–7414.
Michel S, Kloor M, Singh S, Gdynia G, Roth W, von Knebel Doeberitz M et al Coding microsatellite instability analysis in microsatellite unstable small intestinal adenocarcinomas identifies MARCKS as a common target of inactivation. Mol Carcinog 2010; 49: 175–182.
Woerner SM, Kloor M, Mueller A, Rueschoff J, Friedrichs N, Buettner R et al Microsatellite instability of selective target genes in HNPCC-associated colon adenomas. Oncogene 2005; 24: 2525–2535.
Kim NG, Rhee H, Li LS, Kim H, Lee JS, Kim JH et al Identification of MARCKS, FLJ11383 and TAF1B as putative novel target genes in colorectal carcinomas with microsatellite instability. Oncogene 2002; 21: 5081–5087.
Hoffmeister M, Blaker H, Kloor M, Roth W, Toth C, Herpel E et al Body mass index and microsatellite instability in colorectal cancer: a population-based study. Cancer Epidemiol Biomarkers Prev 2013; 22: 2303–2311.
Koh KH, Kang HJ, Li LS, Kim NG, You KT, Yang E et al Impaired nonhomologous end-joining in mismatch repair-deficient colon carcinomas. Lab Invest 2005; 85: 1130–1138.
Shin N, You KT, Lee H, Kim WK, Song M, Choi HJ et al Identification of frequently mutated genes with relevance to nonsense mediated mRNA decay in the high microsatellite instability cancers. Int J Cancer 2011; 128: 2872–2880.
Graff JM, Stumpo DJ, Blackshear PJ . Characterization of the phosphorylation sites in the chicken and bovine myristoylated alanine-rich C kinase substrate protein, a prominent cellular substrate for protein kinase C. J Biol Chem 1989; 264: 11912–11919.
Sundaram M, Cook HW, Byers DM . The MARCKS family of phospholipid binding proteins: regulation of phospholipase D and other cellular components. Biochem Cell Biol 2004; 82: 191–200.
Estrada-Bernal A, Gatlin JC, Sunpaweravong S, Pfenninger KH . Dynamic adhesions and MARCKS in melanoma cells. J Cell Sci 2009; 122: 2300–2310.
Jarboe JS, Anderson JC, Duarte CW, Mehta T, Nowsheen S, Hicks PH et al MARCKS regulates growth and radiation sensitivity and is a novel prognostic factor for glioma. Clin Cancer Res 2012; 18: 3030–3041.
Jin Cho S, La M, Ahn JK, Meadows GG, Joe CO . Tob-mediated cross-talk between MARCKS phosphorylation and ErbB-2 activation. Biochem Biophys Res Commun 2001; 283: 273–277.
Techasen A, Loilome W, Namwat N, Takahashi E, Sugihara E, Puapairoj A et al Myristoylated alanine-rich C kinase substrate phosphorylation promotes cholangiocarcinoma cell migration and metastasis via the protein kinase C-dependent pathway. Cancer Sci 2010; 101: 658–665.
Zhu Y, Dong Q, Tan BJ, Lim WG, Zhou S, Duan W . The PKCalpha-D294G mutant found in pituitary and thyroid tumors fails to transduce extracellular signals. Cancer Res 2005; 65: 4520–4524.
Yamaguchi T, Iijima T, Mori T, Takahashi K, Matsumoto H, Miyamoto H et al Accumulation profile of frameshift mutations during development and progression of colorectal cancer from patients with hereditary nonpolyposis colorectal cancer. Dis Colon Rectum 2006; 49: 399–406.
Rombouts K, Carloni V, Mello T, Omenetti S, Galastri S, Madiai S et al Myristoylated alanine-rich protein kinase C substrate (MARCKS) expression modulates the metastatic phenotype in human and murine colon carcinoma in vitro and in vivo. Cancer Lett 2013; 333: 244–252.
Chen X, Rotenberg SA . PhosphoMARCKS drives motility of mouse melanoma cells. Cell Signal 2010; 22: 1097–1103.
Myat MM, Anderson S, Allen LA, Aderem A . MARCKS regulates membrane ruffling and cell spreading. Curr Biol 1997; 7: 611–614.
Wang J, Gambhir A, Hangyas-Mihalyne G, Murray D, Golebiewska U, McLaughlin S . Lateral sequestration of phosphatidylinositol 4,5-bisphosphate by the basic effector domain of myristoylated alanine-rich C kinase substrate is due to nonspecific electrostatic interactions. J Biol Chem 2002; 277: 34401–34412.
Glaser M, Wanaski S, Buser CA, Boguslavsky V, Rashidzada W, Morris A et al Myristoylated alanine-rich C kinase substrate (MARCKS) produces reversible inhibition of phospholipase C by sequestering phosphatidylinositol 4,5-bisphosphate in lateral domains. J Biol Chem 1996; 271: 26187–26193.
Smyth MJ, Cretney E, Takeda K, Wiltrout RH, Sedger LM, Kayagaki N et al Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) contributes to interferon gamma-dependent natural killer cell protection from tumor metastasis. J Exp Med 2001; 193: 661–670.
Takeda K, Smyth MJ, Cretney E, Hayakawa Y, Kayagaki N, Yagita H et al Critical role for tumor necrosis factor-related apoptosis-inducing ligand in immune surveillance against tumor development. J Exp Med 2002; 195: 161–169.
Kriegl L, Jung A, Horst D, Rizzani A, Jackstadt R, Hermeking H et al Microsatellite instability, KRAS mutations and cellular distribution of TRAIL-receptors in early stage colorectal cancer. PLoS One 2012; 7: e51654.
Johnstone RW, Frew AJ, Smyth MJ . The TRAIL apoptotic pathway in cancer onset, progression and therapy. Nat Rev Cancer 2008; 8: 782–798.
Honing S, Ricotta D, Krauss M, Spate K, Spolaore B, Motley A et al Phosphatidylinositol-(4,5)-bisphosphate regulates sorting signal recognition by the clathrin-associated adaptor complex AP2. Mol Cell 2005; 18: 519–531.
Su R, Han ZY, Fan JP, Zhang YL . A possible role of myristoylated alanine-rich C kinase substrate in endocytic pathway of Alzheimer's disease. Neurosci Bull 2010; 26: 338–344.
Holz RW, Hlubek MD, Sorensen SD, Fisher SK, Balla T, Ozaki S et al A pleckstrin homology domain specific for phosphatidylinositol 4, 5-bisphosphate (PtdIns-4,5-P2) and fused to green fluorescent protein identifies plasma membrane PtdIns-4,5-P2 as being important in exocytosis. J Biol Chem 2000; 275: 17878–17885.
Zhang XD, Franco AV, Nguyen T, Gray CP, Hersey P . Differential localization and regulation of death and decoy receptors for TNF-related apoptosis-inducing ligand (TRAIL) in human melanoma cells. J Immunol 2000; 164: 3961–3970.
Bennett M, Macdonald K, Chan SW, Luzio JP, Simari R, Weissberg P . Cell surface trafficking of Fas: a rapid mechanism of p53-mediated apoptosis. Science 1998; 282: 290–293.
Jones SJ, Ledgerwood EC, Prins JB, Galbraith J, Johnson DR, Pober JS et al TNF recruits TRADD to the plasma membrane but not the trans-Golgi network, the principal subcellular location of TNF-R1. J Immunol 1999; 162: 1042–1048.
Nie Z, Hirsch DS, Luo R, Jian X, Stauffer S, Cremesti A et al A BAR domain in the N terminus of the Arf GAP ASAP1 affects membrane structure and trafficking of epidermal growth factor receptor. Curr Biol 2006; 16: 130–139.
Simova S, Klima M, Cermak L, Sourkova V, Andera L . Arf and Rho GAP adapter protein ARAP1 participates in the mobilization of TRAIL-R1/DR4 to the plasma membrane. Apoptosis 2008; 13: 423–436.
Miura K, Jacques KM, Stauffer S, Kubosaki A, Zhu K, Hirsch DS et al ARAP1: a point of convergence for Arf and Rho signaling. Mol Cell 2002; 9: 109–119.
Morton LA, Yang H, Saludes JP, Fiorini Z, Beninson L, Chapman ER et al MARCKS-ED peptide as a curvature and lipid sensor. ACS Chem Biol 2013; 8: 218–225.
Peuhu E, Rivero-Muller A, Stykki H, Torvaldson E, Holmbom T, Eklund P et al Inhibition of Akt signaling by the lignan matairesinol sensitizes prostate cancer cells to TRAIL-induced apoptosis. Oncogene 2010; 29: 898–908.
Graff JM, Rajan RR, Randall RR, Nairn AC, Blackshear PJ . Protein kinase C substrate and inhibitor characteristics of peptides derived from the myristoylated alanine-rich C kinase substrate (MARCKS) protein phosphorylation site domain. J Biol Chem 1991; 266: 14390–14398.
Li Y, Martin LD, Spizz G, Adler KB . MARCKS protein is a key molecule regulating mucin secretion by human airway epithelial cells in vitro. J Biol Chem 2001; 276: 40982–40990.
Morash SC, Douglas D, McMaster CR, Cook HW, Byers DM . Expression of MARCKS effector domain mutants alters phospholipase D activity and cytoskeletal morphology of SK-N-MC neuroblastoma cells. Neurochem Res 2005; 30: 1353–1364.
Castro F, Dirks WG, Fahnrich S, Hotz-Wagenblatt A, Pawlita M, Schmitt M . High-throughput SNP-based authentication of human cell lines. Int J Cancer 2013; 132: 308–314.
Schmitt M, Pawlita M . High-throughput detection and multiplex identification of cell contaminations. Nucleic Acids Res 2009; 37: e119.
Fassl A, Tagscherer KE, Richter J, Berriel Diaz M, Alcantara Llaguno SR, Campos B et al Notch1 signaling promotes survival of glioblastoma cells via EGFR-mediated induction of anti-apoptotic Mcl-1. Oncogene 2012; 31: 4698–4708.
Brenner H, Chang-Claude J, Seiler CM, Rickert A, Hoffmeister M . Protection from colorectal cancer after colonoscopy: a population-based, case-control study. Ann Intern Med 2011; 154: 22–30.
Lilla C, Verla-Tebit E, Risch A, Jager B, Hoffmeister M, Brenner H et al Effect of NAT1 and NAT2 genetic polymorphisms on colorectal cancer risk associated with exposure to tobacco smoke and meat consumption. Cancer Epidemiol Biomarkers Prev 2006; 15: 99–107.
Rudolph A, Toth C, Hoffmeister M, Roth W, Herpel E, Jansen L et al Expression of oestrogen receptor beta and prognosis of colorectal cancer. Br J Cancer 2012; 107: 831–839.
Findeisen P, Kloor M, Merx S, Sutter C, Woerner SM, Dostmann N et al T25 repeat in the 3' untranslated region of the CASP2 gene: a sensitive and specific marker for microsatellite instability in colorectal cancer. Cancer Res 2005; 65: 8072–8078.
Acknowledgements
We thank the study participants and the interviewers who collected the data. We also greatly appreciate the help of the hospitals, pathology departments and cooperating institutions in recruiting patients for this study and providing tumor samples. We thank Jutta Richter, Ute Handte-Daub, Bettina Walter, Barbara Schreiber and Marina Gernold for their excellent technical assistance. H Bläker has been supported by a grant from the German Research Council (Deutsche Forschungsgemeinschaft; grant number: BL554/3-2). The DACHS study was supported by grants from the German Research Council (Deutsche Forschungsgemeinschaft, grant numbers BR 1704/6-1, BR 1704/6-3, BR 1704/6-4 and CH 390 117/1-1), the German Federal Ministry of Education and Research (grant numbers 01KH0404 and 01ER0814), and the Interdisciplinary Research Program of the National Center for Tumor Diseases (NCT), Heidelberg, Germany.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Bickeböller, M., Tagscherer, K., Kloor, M. et al. Functional characterization of the tumor-suppressor MARCKS in colorectal cancer and its association with survival. Oncogene 34, 1150–1159 (2015). https://doi.org/10.1038/onc.2014.40
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2014.40
This article is cited by
-
Antler-derived microRNA PC-5p-1090 inhibits HCC cell proliferation, migration, and invasion by targeting MARCKS, SMARCAD1, and SOX9
Functional & Integrative Genomics (2023)
-
Pathophysiological roles of myristoylated alanine-rich C-kinase substrate (MARCKS) in hematological malignancies
Biomarker Research (2021)
-
Restoration of MARCK enhances chemosensitivity in cancer
Journal of Cancer Research and Clinical Oncology (2020)
-
MARCKS regulates tonic and chronic active B cell receptor signaling
Leukemia (2019)
-
Upregulation of MARCKS in kidney cancer and its potential as a therapeutic target
Oncogene (2017)