Abstract
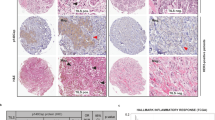
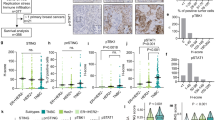
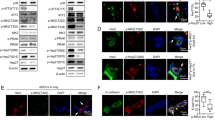
Somatic mutations or deletions of TP53 and PTEN in ductal carcinoma in situ lesions have been implicated in progression to invasive ductal carcinomas. A recent molecular and mutational analysis of breast cancers revealed that inactivation of tumor suppressors, p53 and PTEN, are strongly associated with triple negative breast cancer. In addition, these tumor suppressors have important roles in regulating self-renewal in normal and malignant stem cells. To investigate their role in breast carcinogenesis, we knocked down these genes in human mammary cells and in non-transformed MCF10A cells. p53 and PTEN knockdown synergized to activate pro-inflammatory interleukin-6 (IL6)/Stat3/nuclear factor κB signaling. This resulted in generation of highly metastatic epithelial-to-mesenchymal transition-like cancer stem cells resulting in tumors whose gene expression profile mimicked that found in basal/claudin-low molecular subtype within the triple negative breast tumors. Constitutive activation of this loop in transformed cells was dependent on proteolytic degradation of suppressor of cytokine signaling 3 (SOCS3) resulting in low levels of this protein in basal/claudin-low cell lines and primary tumors. In non-transformed cells, transient activation of the IL6 inflammatory loop induced SOCS3 expression leading to pathway inactivation. In transformed cells, enforced expression of SOCS3 or interfering with IL6 pathway via IL6R blockade inhibited tumor growth and metastasis in mouse xenograft models. Furthermore, circulating tumor cells were significantly reduced in tumor-bearing animals when treated with anti-IL6R antibodies. These studies uncover important connections between inflammation and carcinogenesis and suggest that blocking pro-inflammatory cytokines may be utilized as an attractive strategy to target triple negative breast tumors, which currently lacks molecularly targeted therapies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Steck PA, Pershouse MA, Jasser SA, Yung WK, Lin H, Ligon AH et al. Identification of a candidate tumour suppressor gene, MMAC1, at chromosome 10q23.3 that is mutated in multiple advanced cancers. Nat Genet 1997; 15: 356–362.
Hollstein M, Rice K, Greenblatt MS, Soussi T, Fuchs R, Sorlie T et al. Database of p53 gene somatic mutations in human tumors and cell lines. Nucleic Acids Res 1994; 22: 3551–3555.
Koboldt DC, Fulton RS, McLellan MD, Schmidt H, Kalicki-Veizer J, McMichael JF et al. Comprehensive molecular portraits of human breast tumours. Nature 2012; 490: 61–70.
Cicalese A, Bonizzi G, Pasi CE, Faretta M, Ronzoni S, Giulini B et al. The tumor suppressor p53 regulates polarity of self-renewing divisions in mammary stem cells. Cell 2009; 138: 1083–1095.
Prat A, Parker JS, Karginova O, Fan C, Livasy C, Herschkowitz JI et al. Phenotypic and molecular characterization of the claudin-low intrinsic subtype of breast cancer. Breast Cancer Res 2010; 12: R68.
Croker BA, Krebs DL, Zhang JG, Wormald S, Willson TA, Stanley EG et al. SOCS3 negatively regulates IL-6 signaling in vivo. Nat Immunol 2003; 4: 540–545.
Dontu G, Abdallah WM, Foley JM, Jackson KW, Clarke MF, Kawamura MJ et al. In vitro propagation and transcriptional profiling of human mammary stem/progenitor cells. Genes Dev 2003; 17: 1253–1270.
Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF . Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA 2003; 100: 3983–3988.
Keller PJ, Lin AF, Arendt LM, Klebba I, Jones AD, Rudnick JA et al. Mapping the cellular and molecular heterogeneity of normal and malignant breast tissues and cultured cell lines. Breast Cancer Res 2010; 12: R87.
Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell 2008; 133: 704–715.
Marotta LL, Almendro V, Marusyk A, Shipitsin M, Schemme J, Walker SR et al. The JAK2/STAT3 signaling pathway is required for growth of CD44(+)CD24(-) stem cell-like breast cancer cells in human tumors. J Clin Invest 2011; 121: 2723–2735.
Dandrea M, Donadelli M, Costanzo C, Scarpa A, Palmieri M . MeCP2/H3meK9 are involved in IL-6 gene silencing in pancreatic adenocarcinoma cell lines. Nucleic Acids Res 2009; 37: 6681–6690.
Korkaya H, Kim GI, Davis A, Malik F, Henry NL, Ithimakin S et al. Activation of an IL6 inflammatory loop mediates trastuzumab resistance in HER2+ breast cancer by expanding the cancer stem cell population. Mol Cell 2012; 47: 570–584.
Li Y, Deuring J, Peppelenbosch MP, Kuipers EJ, de Haar C, van der Woude CJ . IL-6-induced DNMT1 activity mediates SOCS3 promoter hypermethylation in ulcerative colitis-related colorectal cancer. Carcinogenesis 2012; 33: 1889–1896.
Niwa Y, Kanda H, Shikauchi Y, Saiura A, Matsubara K, Kitagawa T et al. Methylation silencing of SOCS-3 promotes cell growth and migration by enhancing JAK/STAT and FAK signalings in human hepatocellular carcinoma. Oncogene 2005; 24: 6406–6417.
Sommer U, Schmid C, Sobota RM, Lehmann U, Stevenson NJ, Johnston JA et al. Mechanisms of SOCS3 phosphorylation upon interleukin-6 stimulation. Contributions of Src- and receptor-tyrosine kinases. J Biol Chem 2005; 280: 31478–31488.
Iliopoulos D, Hirsch HA, Struhl K . An epigenetic switch involving NF-kappaB, Lin28, Let-7 MicroRNA, and IL6 links inflammation to cell transformation. Cell 2009; 139: 693–706.
Yasukawa H, Ohishi M, Mori H, Murakami M, Chinen T, Aki D et al. IL-6 induces an anti-inflammatory response in the absence of SOCS3 in macrophages. Nat Immunol 2003; 4: 551–556.
Ying M, Li D, Yang L, Wang M, Wang N, Chen Y et al. Loss of SOCS3 expression is associated with an increased risk of recurrent disease in breast carcinoma. J Cancer Res Clin Oncol 2010; 136: 1617–1626.
Sasi W, Jiang WG, Sharma A, Mokbel K . Higher expression levels of SOCS 1,3,4,7 are associated with earlier tumour stage and better clinical outcome in human breast cancer. BMC Cancer 2010; 10: 178.
Ohsugi Y, Kishimoto T . The recombinant humanized anti-IL-6 receptor antibody tocilizumab, an innovative drug for the treatment of rheumatoid arthritis. Expert Opin Biol Ther 2008; 8: 669–681.
Creighton CJ, Li X, Landis M, Dixon JM, Neumeister VM, Sjolund A et al. Residual breast cancers after conventional therapy display mesenchymal as well as tumor-initiating features. Proc Natl Acad Sci USA 2009; 106: 13820–13825.
Scheel C, Eaton EN, Li SH, Chaffer CL, Reinhardt F, Kah KJ et al. Paracrine and autocrine signals induce and maintain mesenchymal and stem cell states in the breast. Cell 2011; 145: 926–940.
Yao Z, Fenoglio S, Gao DC, Camiolo M, Stiles B, Lindsted T et al. TGF-beta IL-6 axis mediates selective and adaptive mechanisms of resistance to molecular targeted therapy in lung cancer. Proc Natl Acad Sci USA 2010; 107: 15535–15540.
Sansone P, Storci G, Tavolari S, Guarnieri T, Giovannini C, Taffurelli M et al. IL-6 triggers malignant features in mammospheres from human ductal breast carcinoma and normal mammary gland. J Clin Invest 2007; 117: 3988–4002.
Iliopoulos D, Hirsch HA, Wang G, Struhl K . Inducible formation of breast cancer stem cells and their dynamic equilibrium with non-stem cancer cells via IL6 secretion. Proc Natl Acad Sci USA 2011; 108: 1397–1402.
Barnes PJ, Karin M . Nuclear factor-kappaB: a pivotal transcription factor in chronic inflammatory diseases. N Engl J Med 1997; 336: 1066–1071.
Yoshimura A, Naka T, Kubo M . SOCS proteins, cytokine signalling and immune regulation. Nat Rev Immunol 2007; 7: 454–465.
Babon JJ, Kershaw NJ, Murphy JM, Varghese LN, Laktyushin A, Young SN et al. Suppression of cytokine signaling by SOCS3: characterization of the mode of inhibition and the basis of its specificity. Immunity 2012; 36: 239–250.
Lesina M, Kurkowski MU, Ludes K, Rose-John S, Treiber M, Kloppel G et al. Stat3/Socs3 activation by IL-6 transsignaling promotes progression of pancreatic intraepithelial neoplasia and development of pancreatic cancer. Cancer Cell 2011; 19: 456–469.
Ogata H, Kobayashi T, Chinen T, Takaki H, Sanada T, Minoda Y et al. Deletion of the SOCS3 gene in liver parenchymal cells promotes hepatitis-induced hepatocarcinogenesis. Gastroenterology 2006; 131: 179–193.
Korkaya H, Paulson A, Iovino F, Wicha MS . HER2 regulates the mammary stem/progenitor cell population driving tumorigenesis and invasion. Oncogene 2008; 27: 6120–6130.
Korkaya H, Paulson A, Charafe-Jauffret E, Ginestier C, Brown M, Dutcher J et al. Regulation of mammary stem/progenitor cells by PTEN/Akt/beta-catenin signaling. PLoS Biol 2009; 7: e1000121.
Acknowledgements
This work was supported by the NIH grants; CA101860 and CA129765 and Breast Cancer Research Foundation grant to MSW and by the GRU Cancer Center and the Komen grant; KG11230 to HK.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
MSW has financial holdings and is a scientific advisor for OncoMed Pharmaceuticals, is a scientific advisor for Verastem, Paganini and MedImmune and receives research support from Dompe Pharmaceuticals and MedImmune.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Kim, G., Ouzounova, M., Quraishi, A. et al. SOCS3-mediated regulation of inflammatory cytokines in PTEN and p53 inactivated triple negative breast cancer model. Oncogene 34, 671–680 (2015). https://doi.org/10.1038/onc.2014.4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2014.4
Keywords
This article is cited by
-
Pharmacologic inhibition of IL11/STAT3 signaling increases MHC-I expression and T cell infiltration
Journal of Translational Medicine (2023)
-
Tissue levels of suppressor of cytokine signaling-3 (SOCS-3) in mycosis fungoides
Archives of Dermatological Research (2022)
-
Role of inflammatory microenvironment: potential implications for improved breast cancer nano-targeted therapy
Cellular and Molecular Life Sciences (2021)
-
Differential kynurenine pathway metabolism in highly metastatic aggressive breast cancer subtypes: beyond IDO1-induced immunosuppression
Breast Cancer Research (2020)
-
The pleiotropic effects of TNFα in breast cancer subtypes is regulated by TNFAIP3/A20
Oncogene (2019)