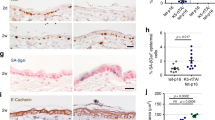
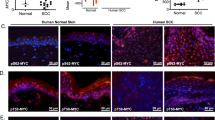
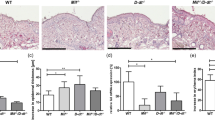
Abstract
Deregulation of matriptase is a consistent feature of human epithelial cancers and correlates with poor disease outcome. We have previously shown that matriptase promotes multi-stage squamous cell carcinogenesis in transgenic mice through dual activation of pro-hepatocyte growth factor–cMet–Akt–mTor proliferation/survival signaling and PAR-2–Gαi–NFκB inflammatory signaling. Matriptase was congenitally and constitutively deregulated in our prior studies, and therefore it was unclear if aberrant matriptase signaling supports only initiation of tumor formation or if it is also critical for the progression of established tumors. To determine this, we here have generated triple-transgenic mice with constitutive deregulation of matriptase and simultaneous inducible expression of the cognate matriptase inhibitor, hepatocyte growth factor inhibitor (HAI)-2. As expected, constitutive expression of HAI-2 suppressed the formation of matriptase-dependent tumors in 7,12-Dimethylbenz(a)anthracene-treated mouse skin. Interestingly, however, the induction of HAI-2 expression in already established tumors markedly impaired malignant progression and caused regression of individual tumors. Tumor regression correlated with reduced accumulation of tumor-associated inflammatory cells, likely caused by diminished expression of pro-tumorigenic inflammatory cytokines. The data suggest that matriptase-dependent signaling may be a therapeutic target for both squamous cell carcinoma chemoprevention and for the treatment of established tumors.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Cheng MF, Huang MS, Lin CS, Lin LH, Lee HS, Jiang JC et al. Expression of matriptase correlates with tumour progression and clinical prognosis in oral squamous cell carcinoma. Histopathology 2014; 65: 24–34.
Ha SY, Kim KY, Lee NK, Kim MG, Kim SH . Overexpression of matriptase correlates with poor prognosis in esophageal squamous cell carcinoma. Virchows Arch 2014; 464: 19–27.
Baba T, Kawaguchi M, Fukushima T, Sato Y, Orikawa H, Yorita K et al. Loss of membrane-bound serine protease inhibitor HAI-1 induces oral squamous cell carcinoma cells' invasiveness. J Pathol 2012; 228: 181–192.
Bergum C, List K . Loss of the matriptase inhibitor HAI-2 during prostate cancer progression. Prostate 2010; 70: 1422–1428.
Bocheva G, Rattenholl A, Kempkes C, Goerge T, Lin CY, D'Andrea MR et al. Role of matriptase and proteinase-activated receptor-2 in nonmelanoma skin cancer. J Invest Dermatol 2009; 129: 1816–1823.
Nakamura K, Abarzua F, Hongo A, Kodama J, Nasu Y, Kumon H et al. Hepatocyte growth factor activator inhibitor-2 (HAI-2) is a favorable prognosis marker and inhibits cell growth through the apoptotic pathway in cervical cancer. Ann Oncol 2009; 20: 63–70.
Jin JS, Cheng TF, Tsai WC, Sheu LF, Chiang H, Yu CP . Expression of the serine protease, matriptase, in breast ductal carcinoma of Chinese women: correlation with clinicopathological parameters. Histol Histopathol 2007; 22: 305–309.
Vogel LK, Saebo M, Skjelbred CF, Abell K, Pedersen ED, Vogel U et al. The ratio of Matriptase/HAI-1 mRNA is higher in colorectal cancer adenomas and carcinomas than corresponding tissue from control individuals. BMC Cancer 2006; 6: 176.
Saleem M, Adhami VM, Zhong W, Longley BJ, Lin CY, Dickson RB et al. A novel biomarker for staging human prostate adenocarcinoma: overexpression of matriptase with concomitant loss of its inhibitor, hepatocyte growth factor activator inhibitor-1. Cancer Epidemiol Biomarkers Prev 2006; 15: 217–227.
Jin JS, Hsieh DS, Loh SH, Chen A, Yao CW, Yen CY . Increasing expression of serine protease matriptase in ovarian tumors: tissue microarray analysis of immunostaining score with clinicopathological parameters. Mod Pathol 2006; 19: 447–452.
Zeng L, Cao J, Zhang X . Expression of serine protease SNC19/matriptase and its inhibitor hepatocyte growth factor activator inhibitor type 1 in normal and malignant tissues of gastrointestinal tract. World J Gastroenterol 2005; 11: 6202–6207.
Tanimoto H, Shigemasa K, Tian X, Gu L, Beard JB, Sawasaki T et al. Transmembrane serine protease TADG-15 (ST14/Matriptase/MT-SP1): expression and prognostic value in ovarian cancer. Br J Cancer 2005; 92: 278–283.
Hoang CD, D'Cunha J, Kratzke MG, Casmey CE, Frizelle SP, Maddaus MA et al. Gene expression profiling identifies matriptase overexpression in malignant mesothelioma. Chest 2004; 125: 1843–1852.
Santin AD, Cane S, Bellone S, Bignotti E, Palmieri M, De Las Casas LE et al. The novel serine protease tumor-associated differentially expressed gene-15 (matriptase/MT-SP1) is highly overexpressed in cervical carcinoma. Cancer 2003; 98: 1898–1904.
Kang JY, Dolled-Filhart M, Ocal IT, Singh B, Lin C-Y, Dickson RB et al. Tissue microarray analysis of HGF/met pathway components reveals a role for Met, matriptase, and HAI-1 in the progression of node-negative breast cancer. Cancer Res 2003; 63: 1101–1105.
Oberst MD, Johnson MD, Dickson RB, Lin CY, Singh B, Stewart M et al. Expression of the serine protease matriptase and its inhibitor HAI-1 in epithelial ovarian cancer: correlation with clinical outcome and tumor clinicopathological parameters. Clin Cancer Res 2002; 8: 1101–1107.
Chou FP, Chen YW, Zhao XF, Xu-Monette ZY, Young KH, Gartenhaus RB et al. Imbalanced matriptase pericellular proteolysis contributes to the pathogenesis of malignant B-cell lymphomas. Am J Pathol 2013; 183: 1306–1317.
Ye j, kawaguchi M, Haruyama y, Kanemaru a, Fukushima y, Yamamoto K et al. Loss of hepatocyte growth factor activator inhibitor type 1 participates in metastatic spreading of human pancreatic cancer cells in a mouse orthotopic transplantation model. Cancer Sci 2014; 105: 44–51.
Tsai CH, Teng CH, Tu YT, Cheng TS, Wu SR, Ko CJ et al. HAI-2 suppresses the invasive growth and metastasis of prostate cancer through regulation of matriptase. Oncogene 2013; 33: 4643–4652.
Wu SR, Cheng TS, Chen WC, Shyu HY, Ko CJ, Huang HP et al. Matriptase is involved in ErbB-2-induced prostate cancer cell invasion. Am J Pathol 2010; 177: 3145–3158.
Cheng H, Fukushima T, Takahashi N, Tanaka H, Kataoka H . Hepatocyte growth factor activator inhibitor type 1 regulates epithelial to mesenchymal transition through membrane-bound serine proteinases. Cancer Res 2009; 69: 1828–1835.
List K, Szabo R, Molinolo A, Sriuranpong V, Redeye V, Murdock T et al. Deregulated matriptase causes ras-independent multistage carcinogenesis and promotes ras-mediated malignant transformation. Genes Dev 2005; 19: 1934–1950.
Yuspa SH . The pathogenesis of squamous cell cancer: lessons learned from studies of skin carcinogenesis. J Dermatol Sci 1998; 17: 1–7.
Szabo R, Hobson JP, Christoph K, Kosa P, List K, Bugge TH . Regulation of cell surface protease matriptase by HAI2 is essential for placental development, neural tube closure and embryonic survival in mice. Development 2009; 136: 2653–2663.
Szabo R, Hobson JP, List K, Molinolo A, Lin CY, Bugge TH . Potent inhibition and global co-localization implicate the transmembrane Kunitz-type serine protease inhibitor hepatocyte growth factor activator inhibitor-2 in the regulation of epithelial matriptase activity. J Biol Chem 2008; 283: 29495–29504.
Sales KU, Friis S, Konkel JE, Godiksen S, Hansen KK, Szabo R et al. Non-hematopoietic PAR-2 is essential for matriptase-driven pre-malignant progression and potentiation of ras-mediated squamous cell carcinogenesis. Oncogene (e-pub ahead of print 27 January 2014; doi:10.1038/onc.2013.563).
Szabo R, Rasmussen AL, Moyer AB, Kosa P, Schafer J, Molinolo A et al. c-Met-induced epithelial carcinogenesis is initiated by the serine protease matriptase. Oncogene 2011; 30: 2003–2016.
Gossen M, Bujard H . Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc Natl Acad Sci USA 1992; 89: 5547–5551.
Vitale-Cross L, Amornphimoltham P, Fisher G, Molinolo AA, Gutkind JS . Conditional expression of K-ras in an epithelial compartment that includes the stem cells is sufficient to promote squamous cell carcinogenesis. Cancer Res 2004; 64: 8804–8807.
Lopez-Otin C, Bond JS . Proteases: multifunctional enzymes in life and disease. J Biol Chem 2008; 283: 30433–30437.
Balmain A, Pragnell IB . Mouse skin carcinomas induced in vivo by chemical carcinogens have a transforming Harvey-ras oncogene. Nature 1983; 303: 72–74.
Balmain A . Transforming ras oncogenes and multistage carcinogenesis. Br J Cancer 1985; 51: 1–7.
Quintanilla M, Brown K, Ramsden M, Balmain A . Carcinogen-specific mutation and amplification of Ha-ras during mouse skin carcinogenesis. Nature 1986; 322: 78–80.
Ise K, Nakamura K, Nakao K, Shimizu S, Harada H, Ichise T et al. Targeted deletion of the H-ras gene decreases tumor formation in mouse skin carcinogenesis. Oncogene 2000; 19: 2951–2956.
Bhatt AS, Welm A, Farady CJ, Vasquez M, Wilson K, Craik CS . Coordinate expression and functional profiling identify an extracellular proteolytic signaling pathway. Proc Natl Acad Sci USA 2007; 104: 5771–5776.
Takeuchi T, Harris JL, Huang W, Yan KW, Coughlin SR, Craik CS . Cellular localization of membrane-type serine protease 1 and identification of protease-activated receptor-2 and single-chain urokinase-type plasminogen activator as substrates. J Biol Chem 2000; 275: 26333–26342.
Lee SL, Dickson RB, Lin CY . Activation of hepatocyte growth factor and urokinase/plasminogen activator by matriptase, an epithelial membrane serine protease. J Biol Chem 2000; 275: 36720–36725.
Kilpatrick LM, Harris RL, Owen KA, Bass R, Ghorayeb C, Bar-Or A et al. Initiation of plasminogen activation on the surface of monocytes expressing the type II transmembrane serine protease matriptase. Blood 2006; 108: 2616–2623.
Bhatt AS, Erdjument-Bromage H, Tempst P, Craik CS, Moasser MM . Adhesion signaling by a novel mitotic substrate of src kinases. Oncogene 2005; 24: 5333–5343.
Ustach CV, Huang W, Conley-LaComb MK, Lin CY, Che M, Abrams J et al. A novel signaling axis of matriptase/PDGF-D/ss-PDGFR in human prostate cancer. Cancer Res 2010; 70: 9631–9640.
Enyedy IJ, Lee SL, Kuo AH, Dickson RB, Lin CY, Wang S . Structure-based approach for the discovery of bis-benzamidines as novel inhibitors of matriptase. J Med Chem 2001; 44: 1349–1355.
Long Y, Lee S, Lin C, Enyedy IJ, Wang S, Li P et al. Synthesis and evaluation of the sunflower derived trypsin inhibitor as a potent inhibitor of the type II transmembrane serine protease, matriptase. Bioorg Med Chem Lett 2001; 11: 2515–2519.
Sun J, Pons J, Craik CS . Potent and selective inhibition of membrane-type serine protease 1 by human single-chain antibodies. Biochemistry 2003; 42: 892–900.
Galkin AV, Mullen L, Fox WD, Brown J, Duncan D, Moreno O et al. CVS-3983, a selective matriptase inhibitor, suppresses the growth of androgen independent prostate tumor xenografts. Prostate 2004; 61: 228.
Forbs D, Thiel S, Stella MC, Sturzebecher A, Schweinitz A, Steinmetzer T et al. In vitro inhibition of matriptase prevents invasive growth of cell lines of prostate and colon carcinoma. Int J Oncol 2005; 27: 1061–1070.
Desilets A, Longpre J-M, Beaulieu M-E, Leduc R . Inhibition of human matriptase by eglin c variants. FEBS Lett 2006; 580: 2227–2232.
Quimbar P, Malik U, Sommerhoff CP, Kaas Q, Chan LY, Huang Y-H et al. High-affinity cyclic peptide matriptase inhibitors. J Biol Chem 2013; 288: 13885–13896.
Gray K, Elghadban S, Thongyoo P, Owen KA, Szabo R, Bugge TH et al. Potent and specific inhibition of the biological activity of the type-II transmembrane serine protease matriptase by the cyclic microprotein MCoTI-II. Thromb Haemost 2014; 112: 402–411.
Murillas R, Larcher F, Conti CJ, Santos M, Ullrich A, Jorcano JL . Expression of a dominant negative mutant of epidermal growth factor receptor in the epidermis of transgenic mice elicits striking alterations in hair follicle development and skin structure. EMBO J 1995; 14: 5216–5223.
Acknowledgements
We thank Dr Mary Jo Danton and Dr J Silvio Gutkind for critically reviewing this manuscript. We also thank Andrew Cho and Dr Ashok Kulkarni, NIDCR Gene Targeting Facility, for generation of transgenic mice. Histology was performed by Histoserv, Inc., Germantown, MD, USA. The study was supported by the NIDCR Intramural Research Program (THB), Sao Paulo Research Foundation 2014/14311-0 (KUS), The Harboe Foundation, The Foundation of 17.12.1981 and the Lundbeck Foundation (SF).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Sales, K., Friis, S., Abusleme, L. et al. Matriptase promotes inflammatory cell accumulation and progression of established epidermal tumors. Oncogene 34, 4664–4672 (2015). https://doi.org/10.1038/onc.2014.391
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2014.391
This article is cited by
-
Cell surface–anchored serine proteases in cancer progression and metastasis
Cancer and Metastasis Reviews (2019)
-
Intestinal regulation of suppression of tumorigenicity 14 (ST14) and serine peptidase inhibitor, Kunitz type -1 (SPINT1) by transcription factor CDX2
Scientific Reports (2018)