Abstract
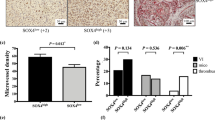
Hepatocellular carcinoma (HCC) is one of the leading malignancies worldwide. Myocyte enhancer factor 2C (MEF2C) was traditionally regarded as a development-associated factor and was recently reported to be an oncogene candidate. We have previously reported overexpression of MEF2C in HCC; however, the roles of MEF2C in HCC remain to be clarified. In this study, HCC cell lines and a xenograft mouse model were used to determine the functions of MEF2C in vitro and in vivo, respectively. Specific plasmids and small interfering RNA were used to upregulate and downregulate MEF2C expression, respectively. Functional assays were performed to assess the influence of MEF2C on cell proliferation, and VEGF-induced vasculogenic mimicry, migration/invasion as well as angiogenesis. Co-immunoprecipitation was conducted to identify the interaction of MEF2C and β-catenin. Human HCC tissue microarrays were used to investigate correlations among MEF2C, β-catenin and involved biomarkers. MEF2C was found to mediate VEGF-induced vasculogenic mimicry, angiogenesis and migration/invasion, with involvement of the p38 MAPK and PKC signaling pathways. However, MEF2C itself inhibited tumor growth in vitro and in vivo. MEF2C was upregulated by and directly interacted with β-catenin. The nuclear translocation of β-catenin blocked by MEF2C was responsible for MEF2C-mediated growth inhibition. The nuclear translocation of MEF2C was associated with intracellular calcium signaling induced by β-catenin. HCC microarrays showed correlations of nuclear MEF2C with the angiogenesis-associated biomarker, CD31, and cytosolic MEF2C with the proliferation-associated biomarker, Ki-67. MEF2C showed double-edged activities in HCC, namely mediating VEGF-induced malignancy enhancement while inhibiting cancer proliferation via blockade of Wnt/β-catenin signaling. The overall effect of MEF2C in HCC progression regulation was dictated by its subcellular distribution. This should be determined prior to any MEF2C-associated intervention in HCC.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Siegel R, Naishadham D, Jemal A . Cancer statistics, 2013. CA Cancer J Clin 2013; 63: 11–30.
Potthoff MJ, Olson EN . MEF2: a central regulator of diverse developmental programs. Development 2007; 134: 4131–4140.
Homminga I, Pieters R, Langerak AW, de Rooi JJ, Stubbs A, Verstegen M et al. Integrated transcript and genome analyses reveal NKX2-1 and MEF2C as potential oncogenes in T cell acute lymphoblastic leukemia. Cancer Cell 2011; 19: 484–497.
Li BQ, Huang T, Liu L, Cai YD, Chou KC . Identification of colorectal cancer related genes with mRMR and shortest path in protein-protein interaction network. PLoS ONE 2012; 7: e33393.
Zhang JJ, Zhu Y, Xie KL, Peng YP, Tao JQ, Tang J et al. Yin Yang-1 suppresses invasion and metastasis of pancreatic ductal adenocarcinoma by downregulating MMP10 in MUC4/ErbB2/p38/MEF2C-dependent mechanism. Mol Cancer 2014; 13: 130.
Bai X, Wu L, Liang T, Liu Z, Li J, Li D et al. Overexpression of myocyte enhancer factor 2 and histone hyperacetylation in hepatocellular carcinoma. J Cancer Res Clin Oncol 2008; 134: 83–91.
Hoeben A, Landuyt B, Highley MS, Wildiers H, Van Oosterom AT, De Bruijn EA . Vascular endothelial growth factor and angiogenesis. Pharmacol Rev 2004; 56: 549–580.
Maiti D, Xu Z, Duh EJ . Vascular endothelial growth factor induces MEF2C and MEF2-dependent activity in endothelial cells. Invest Ophthalmol Vis Sci 2008; 49: 3640–3648.
Lin Q, Lu J, Yanagisawa H, Webb R, Lyons GE, Richardson JA et al. Requirement of the MADS-box transcription factor MEF2C for vascular development. Development 1998; 125: 4565–4574.
Xu J, Cao S, Wang L, Xu R, Chen G, Xu Q . VEGF promotes the transcription of the human PRL-3 gene in HUVEC through transcription factor MEF2C. PLoS ONE 2011; 6: e27165.
Bi W, Drake CJ, Schwarz JJ . The transcription factor MEF2C-null mouse exhibits complex vascular malformations and reduced cardiac expression of angiopoietin 1 and VEGF. Dev Biol 1999; 211: 255–267.
Shang Y, Doan CN, Arnold TD, Lee S, Tang AA, Reichardt LF et al. Transcriptional corepressors HIPK1 and HIPK2 control angiogenesis via TGF-beta-TAK1-dependent mechanism. PLoS Biol 2013; 11: e1001527.
White BD, Chien AJ, Dawson DW . Dysregulation of Wnt/beta-catenin signaling in gastrointestinal cancers. Gastroenterology 2012; 142: 219–232.
Zhang Q, Bai X, Chen W, Ma T, Hu Q, Liang C et al. Wnt/beta-catenin signaling enhances hypoxia-induced epithelial-mesenchymal transition in hepatocellular carcinoma via crosstalk with hif-1alpha signaling. Carcinogenesis 2013; 34: 962–973.
Saint Just Ribeiro M, Hansson ML, Lindberg MJ, Popko-Scibor AE, Wallberg AE . GSK3beta is a negative regulator of the transcriptional coactivator MAML1. Nucleic Acids Res 2009; 37: 6691–6700.
Riazi AM, Takeuchi JK, Hornberger LK, Zaidi SH, Amini F, Coles J et al. NKX2-5 regulates the expression of beta-catenin and GATA4 in ventricular myocytes. PLoS ONE 2009; 4: e5698.
De Angelis L, Borghi S, Melchionna R, Berghella L, Baccarani-Contri M, Parise F et al. Inhibition of myogenesis by transforming growth factor beta is density-dependent and related to the translocation of transcription factor MEF2 to the cytoplasm. Proc Natl Acad Sci USA 1998; 95: 12358–12363.
Ellis LM, Hicklin DJ . VEGF-targeted therapy: mechanisms of anti-tumour activity. Nat Rev Cancer 2008; 8: 579–591.
Seftor RE, Hess AR, Seftor EA, Kirschmann DA, Hardy KM, Margaryan NV et al. Tumor cell vasculogenic mimicry: from controversy to therapeutic promise. Am J Pathol 2012; 181: 1115–1125.
Kirschmann DA, Seftor EA, Hardy KM, Seftor RE, Hendrix MJ . Molecular pathways: vasculogenic mimicry in tumor cells: diagnostic and therapeutic implications. Clin Cancer Res 2012; 18: 2726–2732.
Goel HL, Mercurio AM . VEGF targets the tumor cells. Nat Rev Cancer 2013; 13: 871–882.
Zhou L, Wang DS, Li QJ, Sun W, Zhang Y, Dou KF . Downregulation of the Notch signaling pathway inhibits hepatocellular carcinoma cell invasion by inactivation of matrix metalloproteinase-2 and -9 and vascular endothelial growth factor. Oncol Rep 2012; 28: 874–882.
Maatta M, Soini Y, Liakka A, Autio-Harmainen H . Differential expression of matrix metalloproteinase (MMP)-2, MMP-9, and membrane type 1-MMP in hepatocellular and pancreatic adenocarcinoma: implications for tumor progression and clinical prognosis. Clin Cancer Res 2000; 6: 2726–2734.
Chen SL, Wang SC, Hosking B, Muscat GE . Subcellular localization of the steroid receptor coactivators (SRCs) and MEF2 in muscle and rhabdomyosarcoma cells. Mol Endocrinol 2001; 15: 783–796.
Borghi S, Molinari S, Razzini G, Parise F, Battini R, Ferrari S . The nuclear localization domain of the MEF2 family of transcription factors shows member-specific features and mediates the nuclear import of histone deacetylase 4. J Cell Sci 2001; 114: 4477–4483.
Evdokimova V, Tognon C, Ng T, Sorensen PH . Reduced proliferation and enhanced migration: two sides of the same coin? Molecular mechanisms of metastatic progression by YB-1. Cell Cycle 2009; 8: 2901–2906.
Hugo HJ, Pereira L, Suryadinata R, Drabsch Y, Gonda TJ, Gunasinghe NP et al. Direct repression of MYB by ZEB1 suppresses proliferation and epithelial gene expression during epithelial-to-mesenchymal transition of breast cancer cells. Breast Cancer Res 2013; 15: R113.
Akhurst RJ, Derynck R . TGF-beta signaling in cancer—a double-edged sword. Trends Cell Biol 2001; 11: S44–S51.
Oka M, Hamamoto Y, Okabe N . Evaluating system for predicting cancer return, Patent Number: CN100398663(C), granted on 02 July 2008.
Liu Y, Sanchez-Tillo E, Lu X, Huang L, Clem B, Telang S et al. Sequential inductions of the ZEB1 transcription factor caused by mutation of Rb and then Ras proteins are required for tumor initiation and progression. J Biol Chem 2013; 288: 11572–11580.
Fearon ER, Vogelstein B . A genetic model for colorectal tumorigenesis. Cell 1990; 61: 759–767.
Jen KY, Song IY, Banta KL, Wu D, Mao JH, Balmain A . Sequential mutations in Notch1, Fbxw7, and Tp53 in radiation-induced mouse thymic lymphomas. Blood 2012; 119: 805–809.
Bienvenu T, Diebold B, Chelly J, Isidor B . Refining the phenotype associated with MEF2C point mutations. Neurogenetics 2013; 14: 71–75.
Novara F, Rizzo A, Bedini G, Girgenti V, Esposito S, Pantaleoni C et al. MEF2C deletions and mutations versus duplications: a clinical comparison. Eur J Med Genet 2013; 56: 260–265.
Yang M, Chen J, Su F, Yu B, Lin L, Liu Y et al. Microvesicles secreted by macrophages shuttle invasion-potentiating microRNAs into breast cancer cells. Mol Cancer 2011; 10: 117.
Zhao JX, Yue WF, Zhu MJ, Du M . AMP-activated protein kinase regulates beta-catenin transcription via histone deacetylase 5. J Biol Chem 2011; 286: 16426–16434.
McGee SL, van Denderen BJ, Howlett KF, Mollica J, Schertzer JD, Kemp BE et al. AMP-activated protein kinase regulates GLUT4 transcription by phosphorylating histone deacetylase 5. Diabetes 2008; 57: 860–867.
Tate CR, Rhodes LV, Segar HC, Driver JL, Pounder FN, Burow ME et al. Targeting triple-negative breast cancer cells with the histone deacetylase inhibitor panobinostat. Breast Cancer Res 2012; 14: R79.
Acknowledgements
We thank Professor Xu Qiang (State Key Laboratory of Pharmaceutical Biotechnology, School of Life Sciences, Nanjing University, China) for the generous gift of MEF2C overexpression plasmid. We appreciate Mr Xie Shangzhi, Mr Hu Liqiang, and Miss Chen Conglin (The Second Affiliated Hospital, Zhejiang University School of Medicine, China) for their great help in certain experiments. This study was financially supported by the National Natural Science Foundation of China (No. 81171884), the National Key Basic Research Program of China (No. 2014CB542101), the Ministry-Province Co-supportive Project of China, and Innovation and High-Level Talent Training Program of Department of Health of Zhejiang.
Author Contributions
XLB, QZ, ZZP and TBL designed the experiments. XLB, QZ, LYY, FL, XS, YC, QDH, QHF, ZC and WS performed the experiments and analyzed the data. TBL supervised the project. XLB, QZ and TBL wrote the report.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Bai, X., Zhang, Q., Ye, L. et al. Myocyte enhancer factor 2C regulation of hepatocellular carcinoma via vascular endothelial growth factor and Wnt/β-catenin signaling. Oncogene 34, 4089–4097 (2015). https://doi.org/10.1038/onc.2014.337
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2014.337
This article is cited by
-
Effects of RNA methylation on Tumor angiogenesis and cancer progression
Molecular Cancer (2023)
-
USP22 upregulates ZEB1-mediated VEGFA transcription in hepatocellular carcinoma
Cell Death & Disease (2023)
-
Progress on the roles of MEF2C in neuropsychiatric diseases
Molecular Brain (2022)
-
MEF2A transcriptionally upregulates the expression of ZEB2 and CTNNB1 in colorectal cancer to promote tumor progression
Oncogene (2021)
-
RNA m6A methylation promotes the formation of vasculogenic mimicry in hepatocellular carcinoma via Hippo pathway
Angiogenesis (2021)