Abstract
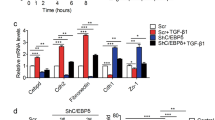
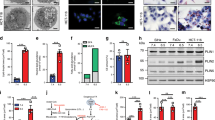
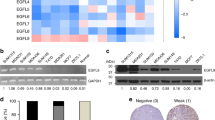
Metastatic progression, including extravasation and micrometastatic outgrowth, is the main cause of cancer patient death. Recent studies suggest that cancer cells reprogram their metabolism to support increased proliferation through increased glycolysis and biosynthetic activities, including lipogenesis pathways. However, metabolic changes during metastatic progression, including alterations in regulatory gene expression, remain undefined. We show that transforming growth factor beta 1 (TGFβ1)-induced epithelial-to-mesenchymal transition (EMT) is accompanied by coordinately reduced enzyme expression required to convert glucose into fatty acids, and concomitant enhanced respiration. Overexpressed Snail1, a transcription factor mediating TGFβ1-induced EMT, was sufficient to suppress carbohydrate-responsive-element-binding protein (ChREBP, a master lipogenic regulator), and fatty acid synthase (FASN), its effector lipogenic gene. Stable FASN knockdown was sufficient to induce EMT, stimulate migration and extravasation in vitro. FASN silencing enhanced lung metastasis and death in vivo. These data suggest that a metabolic transition that suppresses lipogenesis and favors energy production is an essential component of TGFβ1-induced EMT and metastasis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Semenza GL . Molecular mechanisms mediating metastasis of hypoxic breast cancer cells. Trends Mol Med 2012; 18: 534–543.
Pallis AG, Syrigos K . Targeted (and chemotherapeutic) agents as maintenance treatment in patients with metastatic non-small-cell lung cancer: current status and future challenges. Cancer Treat Rev 2012; 38: 861–867.
Zavadil J, Bottinger EP . TGF-beta and epithelial-to-mesenchymal transitions. Oncogene 2005; 24: 5764–5774.
Acloque H, Adams MS, Fishwick K, Bronner-Fraser M, Nieto MA . Epithelial-mesenchymal transitions: the importance of changing cell state in development and disease. J Clin Invest 2009; 119: 1438–1449.
Yang L, Moses HL . Transforming growth factor beta: tumor suppressor or promoter? Are host immune cells the answer? Cancer Res 2008; 68: 9107–9111.
Kim WS, Park C, Jung YS, Kim HS, Han J Park CH et al. Reduced transforming growth factor-beta type II receptor (TGF-beta RII) expression in adenocarcinoma of the lung. Anticancer Res 1999; 19: 301–306.
Elliott RL, Blobe GC . Role of transforming growth factor Beta in human cancer. J Clin Oncol 2005; 23: 2078–2093.
Cano A, Perez-Moreno MA, Rodrigo I, Locascio A, Blanco MJ, del Barrio MG et al. The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat Cell Biol 2000; 2: 76–83.
Batlle E, Sancho E, Franci C, Dominguez D, Monfar M, Baulida J et al. The transcription factor snail is a repressor of E-cadherin gene expression in epithelial tumour cells. Nat Cell Biol 2000; 2: 84–89.
Vander Heiden MG, Cantley LC, Thompson CB . Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science 2009; 324: 1029–1033.
DeBerardinis RJ, Lum JJ, Hatzivassiliou G, Thompson CB . The biology of cancer: metabolic reprogramming fuels cell growth and proliferation. Cell Metab 2008; 7: 11–20.
Shao W, Espenshade PJ . Expanding roles for SREBP in metabolism. Cell Metab 2012; 16: 414–419.
Hanai J, Doro N, Sasaki AT, Kobayashi S, Cantley LC, Seth P et al. Inhibition of lung cancer growth: ATP citrate lyase knockdown and statin treatment leads to dual blockade of mitogen-activated protein kinase (MAPK) and phosphatidylinositol-3-kinase (PI3K)/AKT pathways. J Cell Physiol 2012; 227: 1709–1720.
Hatzivassiliou G, Zhao F, Bauer DE, Andreadis C, Shaw AN, Dhanak D et al. ATP citrate lyase inhibition can suppress tumor cell growth. Cancer Cell 2005; 8: 311–321.
Bauer DE, Hatzivassiliou G, Zhao F, Andreadis C, Thompson CB . ATP citrate lyase is an important component of cell growth and transformation. Oncogene 2005; 24: 6314–6322.
Brusselmans K, De Schrijver E, Verhoeven G, Swinnen JV . RNA interference-mediated silencing of the acetyl-CoA-carboxylase-alpha gene induces growth inhibition and apoptosis of prostate cancer cells. Cancer Res 2005; 65: 6719–6725.
Lupu R, Menendez JA . Pharmacological inhibitors of Fatty Acid Synthase (FASN)—catalyzed endogenous fatty acid biogenesis: a new family of anti-cancer agents? Curr Pharm Biotechnol 2006; 7: 483–493.
Kim JH, Jang YS, Eom KS, Hwang YI, Kang HR, Jang SH et al. Transforming growth factor beta1 induces epithelial-to-mesenchymal transition of A549 cells. J Korean Med Sci 2007; 22: 898–904.
Araki S, Eitel JA, Batuello CN, Bijangi-Vishehsaraei K, Xie XJ, Danielpour D et al. TGF-beta1-induced expression of human Mdm2 correlates with late-stage metastatic breast cancer. J Clin Invest 2010; 120: 290–302.
Liu X, Sun H, Qi J, Wang L, He S, Liu J et al. Sequential introduction of reprogramming factors reveals a time-sensitive requirement for individual factors and a sequential EMT-MET mechanism for optimal reprogramming. Nat Cell Biol 2013; 15: 829–838.
Uyeda K, Yamashita H, Kawaguchi T . Carbohydrate responsive element-binding protein (ChREBP): a key regulator of glucose metabolism and fat storage. Biochem Pharmacol 2002; 63: 2075–2080.
De Schrijver E, Brusselmans K, Heyns W, Verhoeven G, Swinnen JV . RNA interference-mediated silencing of the fatty acid synthase gene attenuates growth and induces morphological changes and apoptosis of LNCaP prostate cancer cells. Cancer Res 2003; 63: 3799–3804.
Ferreira LM, Hebrant A, Dumont JE . Metabolic reprogramming of the tumor. Oncogene 2012; 31: 3999–4011.
Metallo CM, Vander Heiden MG . Understanding metabolic regulation and its influence on cell physiology. Mol Cell 2013; 49: 388–398.
Zhou H, Zhang B, Zheng J, Yu M, Zhou T, Zhao K et al. The inhibition of migration and invasion of cancer cells by graphene via the impairment of mitochondrial respiration. Biomaterials 2014; 35: 1597–1607.
Yuan HX, Xiong Y, Guan KL . Nutrient sensing, metabolism, and cell growth control. Mol Cell 2013; 49: 379–387.
Eberle D, Hegarty B, Bossard P, Ferre P, Foufelle F . SREBP transcription factors: master regulators of lipid homeostasis. Biochimie 2004; 86: 839–848.
Thompson CB . Metabolic enzymes as oncogenes or tumor suppressors. N Engl J Med 2009; 360: 813–815.
Ikushima H, Miyazono K . TGFbeta signalling: a complex web in cancer progression. Nat Rev Cancer 2010; 10: 415–424.
Huang X, Dong Y, Bey EA, Kilgore JA, Bair JS, Li LS et al. An NQO1 substrate with potent antitumor activity that selectively kills by PARP1-induced programmed necrosis. Cancer Res 2012; 72: 3038–3047.
Goetz EM, Shankar B, Zou Y, Morales JC, Luo X, Araki S et al. ATM-dependent IGF-1 induction regulates secretory clusterin expression after DNA damage and in genetic instability. Oncogene 2011; 30: 3745–3754.
Klokov D, Leskov K, Araki S, Zou Y, Goetz EM, Luo X et al. Low dose IR-induced IGF-1-sCLU expression: a p53-repressed expression cascade that interferes with TGFbeta1 signaling to confer a pro-survival bystander effect. Oncogene 2013; 32: 479–490.
Kurosu H, Choi M, Ogawa Y, Dickson AS, Goetz R, Eliseenkova AV et al. Tissue-specific expression of betaKlotho and fibroblast growth factor (FGF) receptor isoforms determines metabolic activity of FGF19 and FGF21. J Biol Chem 2007; 282: 26687–26695.
Acknowledgements
We are grateful to Dr Xiuquan Luo for his aid in the BLI measurements and animal injections. This work was supported by DOE/NASA grant DE-FG-022179-18-21 and by NIH R01 CA139217 to DAB and NIH RO1 CA157996 and CPRIT RP130272 to RJD. We are grateful to the imaging core of the Simmons Cancer Center Support Grant (5P30 CA142543-03).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Jiang, L., Xiao, L., Sugiura, H. et al. Metabolic reprogramming during TGFβ1-induced epithelial-to-mesenchymal transition. Oncogene 34, 3908–3916 (2015). https://doi.org/10.1038/onc.2014.321
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2014.321
This article is cited by
-
Oxidative stress regulation and related metabolic pathways in epithelial–mesenchymal transition of breast cancer stem cells
Stem Cell Research & Therapy (2023)
-
Signaling pathways in cancer metabolism: mechanisms and therapeutic targets
Signal Transduction and Targeted Therapy (2023)
-
Retrograde regulation of mitochondrial fission and epithelial to mesenchymal transition in hepatocellular carcinoma by GCN5L1
Oncogene (2023)
-
Synergistic effects of ISL1 and KDM6B on non-alcoholic fatty liver disease through the regulation of SNAI1
Molecular Medicine (2022)
-
CHREBP suppresses gastric cancer progression via the cyclin D1-Rb-E2F1 pathway
Cell Death Discovery (2022)