Abstract
Dapper homolog (DACT) 2 is one of the Dact gene family members, which are important modulators of Wnt signaling pathway. We aim to clarify its epigenetic inactivation, biological function and clinical implication in colon cancer. DACT2 was silenced in five out of eight colon cancer cell lines, but robustly expressed in normal colon tissues. The loss of DACT2 expression was regulated by promoter hypermethylation. Restoring DACT2 expression in colon cancer cell lines suppressed tumor cell growth by inducing cell apoptosis and inhibiting cell proliferation both in vitro and in vivo. Moreover, DACT2 overexpression effectively reduced lung metastasis of colon cancer cells in nude mice. These effects by DACT2 were attributed to inhibition of Wnt/β-catenin signaling. Reexpression of DACT2 significantly suppressed the transcriptional activity of both wild-type β-catenin and degradation-resistant form mutant β-catenin (S33Y). DACT2 could actively shuttle into and out of nuclei, with its predominant steady-state localization in the cytoplasm dependent on its nuclear export signal. Co-immunoprecipitation results indicated that DACT2 strongly associated β-catenin as well as lymphoid enhancer-binding factor 1 (LEF1) and directly disrupted the formation of the β-catenin–LEF1 complex in the nucleus. Whereas in the cytoplasm, DACT2 restored junctional localization of E-cadherin–β-catenin complexes and prevented β-catenin nuclear translocation through direct interaction with β-catenin. DACT2 methylation was detected in 43.3% (29/67) of colon cancer tissues, but none in normal controls. Multivariate analysis revealed that patients with DACT2 methylation had a significant decrease in overall survival (P=0.006). Kaplan–Meier survival curves showed that DACT2 methylation was significantly associated with shortened survival in stage I–III colon cancer patients. In conclusion, DACT2 acts as a functional tumor suppressor in colon cancer through inhibiting Wnt/β-catenin signaling. Its methylation at early stages of colon carcinogenesis is an independent prognostic factor.
Similar content being viewed by others
Introduction
Colon cancer is the third most common malignancy worldwide.1 Colon cancer development is a multistep process with both genomic and epigenomic perturbations.2 Aberrant CpG island methylations of genes are accumulated during the multistep pathogenesis of colorectal cancer. Identification of novel genes silenced by promoter methylation may shed new light on the mechanisms for the inactivation of tumor suppressive pathways, which may provide new approaches for tumor diagnostic and therapeutic evaluation.
Dapper homolog (DACT) 2 is one of the Dact gene family members in mammals, which functionally and physically interact with Wnt signaling pathway during vertebrate development.3 Dact family has three members, including DACT1, DACT2 and DACT3. As a context-dependent Wnt regulator, DACT1 could synergize with Disheveled (Dvl) 2 to enhance Wnt/β-catenin activity, whereas in some contexts, it acts as a Wnt inhibitor or has no significant impact on Wnt/β-catenin signaling.3, 4, 5, 6, 7 In comparison with DACT1, DACT2 has been less well characterized, and its role in tumorigenic signaling and development is still largely unclear. Although genetic and epigenetic analyses have supported that DACT2 acts as an antitumor protein through regulating Wnt/β-catenin signaling in lung cancer, the current studies on DACT2 are only limited to brief description of its relationship with Wnt/β-catenin signaling.8 How DACT2 affects Wnt/β-catenin signaling in molecular details and whether this effect is cell type and context dependent are largely unknown. Through a genome-wide screening, we identified that DACT2 was frequently silenced by promoter methylation in colon cancer. However, the role and the clinical implication of DACT2 in colon cancer remain elusive. In this study, the epigenetic regulation, biological function, molecular basis and clinical impact of DACT2 in colon cancer were examined.
Results
Silence or downregulation of DACT2 by promoter methylation in colon cancer
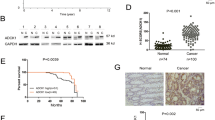
DACT2 was strongly expressed in normal human colon tissue (Figure 1a). In contrast, among eight colon cancer cell lines examined, the mRNA expression of DACT2 was silenced in five cell lines, except SW480, SW620 and Caco-2 (Figure 1a). To examine the contribution of promoter methylation to the downregulation of DACT2, the methylation status of DACT2 promoter was examined by bisulfite genomic sequencing (BGS). Dense promoter methylation was observed in all five silenced colon cancer cell lines, but no methylation was observed in SW480, SW620 and Caco-2, which exhibited DACT2 expression, as well as normal colon tissue (Figure 1c), inferring that transcriptional silence of DACT2 was mediated by promoter methylation in colon cancer cells.
DACT2 was inactivated by promoter methylation in colon cancer. (a) DACT2 was frequently silenced in colon cancer cell lines. The normal colon tissue is from epithelial tissue and is mainly composed of epithelial cells. (b) The CpG island of DACT2. Transcription start site, BGS region and promoter region for evaluation of DACT2 methylation status were indicated. (c) Methylation status of DACT2 promoter was determined by BGS.
DACT2 inhibits colon cancer cell growth and induces cell apoptosis in vitro
To elucidate the function of DACT2 in colon cancer, we examined the effect of DACT2 reexpression on growth characteristics of colon cancer cells by 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) assay. Reexpression of DACT2 in the stable transfected HCT116 and HT-29 cells was confirmed by western blot (Figure 2a). Ectopic expression of DACT2 caused a significant decrease in cell viability in HCT116 (P<0.01) and HT-29 (P<0.01; Figure 2a). On the other hand, small interfering RNA (siRNA)-mediated knockdown of DACT2 in human embryonic kidney cell HEK-293 significantly increased clonogenic ability (P<0.01; Figure 2b). Moreover, ectopic expression of DACT2 led to a significant increase of apoptotic cells in both HCT116 (P<0.01) and HT-29 cells (P<0.01; Figure 2c). Induction of apoptosis was further evidenced by the enhanced cleavage of PARP (poly (ADP-ribose) polymerase) in stably transfected HCT116 and HT-29 cells as compared with controls (Figure 3a).
DACT2 inhibited tumor growth and metastasis. (a) Effect of DACT2 on cell viability was evaluated by MTS assay in HCT116 and HT-29 cells. (b) Effect of DACT2 knockdown on colony formation in an immortalized human embryonic kidney cell HEK-293. (c) Cell apoptosis was examined by flow cytometry analysis of Annexin V-APC and 7-AAD double staining. Upper left region shows the necrotic cells, upper right shows the late apoptotic cells, lower left shows the live cells and lower right shows the early apoptotic cells. The relative proportion of Annexin V-positive cells was and counted as apoptotic cells including early and late apoptotic cells. (d) Representative images of the tumors (arrows) formed in nude mice induced by control HCT116 cells (left dorsal flank) and DACT2-expressing HCT116 cells (right dorsal flank). (e) Subcutaneous tumor growth curve of DACT2-expressing HCT116 cells in nude mice was compared with control cells. The DACT2 group showed a retarded tumor growth compared with the control group (P<0.01). (f) Representative Ki-67 staining in xenograft tumors of nude mice (magnification, × 200). A decrease in the number of Ki-67-positive cells (brown-stained nuclei) was evident in DACT2-expressing tumors (P<0.05). (g) DACT2 suppressed tumor metastasis in vivo. The total number of metastatic nodules was quantified in lungs of nude mice (n=4 per group) 8 weeks after tail vein injection of control (red bars) and DACT2-expressing (blue bars) HCT116 cells. Values for individual mice were shown above the bars; values by group were also denoted. Data are mean±s.d. (h) Representative hematoxylin and eosin-stained lung sections containing metastatic foci.
The antitumor effect of DACT2 was mediated by inhibiting Wnt/β-catenin signaling pathway. (a) The effect of DACT2 overexpression on Wnt/β-catenin signaling, cleaved PARP (poly (ADP-ribose) polymerase) and epithelial marker E-cadherin expression was assessed by western blot. (b) The effect of DACT2 on Wnt/β-catenin signaling pathway was assessed by dual-luciferase reporter assays in HCT116 and HT-29 cells. (c) Depletion of DACT2 enhanced β-catenin-stimulated transcriptional activity. (d) β-catenin immunofluorescent staining demonstrated that β-catenin nuclear translocation was markedly reduced in DACT2-expressing HT-29 cells compared with control cells. Nuclei were counterstained with 4′,6-diamidino-2-phenylindole (DAPI, blue). (e) Reexpression of DACT2 significantly reduced TOPflash activity induced by wild-type β-catenin as well as degradation-resistant β-catenin mutant (S33Y). (f) Double immunofluorescent staining of E-cadherin and Flag-DACT2 was performed in Flag-DACT-transfected HCT116 and HT-29 cells. (g) E-cadherin knockdown partially relieved DACT2-mediated metastasis inhibition.
DACT2 inhibits the invasive and tumorigenic ability of colon cancer cells in vivo
We also tested whether DACT2 could suppress the growth of colon cancer cells in nude mice in vivo. Subcutaneous tumor xenografts of HCT116 cells with stable DACT2 expression in nude mice exhibited a reduced rate of tumor growth compared with control cells (P<0.01; Figures 2d and e). Cell proliferation in the xenograft tumors was examined by Ki-67 staining. Consistent with the result obtained in colon cancer cell lines in vitro, DACT2-expressing tumors displayed significantly less proliferative cells (P<0.05; Figure 2f). To elucidate the anti-invasive function of DACT2 in vivo, an experimental metastasis assay was used to compare the metastatic tumor nodules formed in the lungs of nude mice after tail vein inoculation with DACT2-expressing or control HCT116 cells. At 8 weeks after injection, mice were killed, and the lungs were collected. The total number of metastatic nodules in the lungs was significantly lower in mice injected with DACT2-expressing cells than in mice injected with control cells (57±13 versus 23±14; P<0.01; Figures 2g and h). Therefore, DACT2 had a critical role in colon cancer growth and metastasis.
The tumor suppressive effect of DACT2 is mediated by suppressing Wnt/β-catenin signaling pathway
As DACT2 is a member of the Dact family, which was first identified as an antagonist of Dvl, the central hub of Wnt signaling,9 we evaluated the effect of DACT2 on Wnt/β-catenin signaling pathway by western blot. We found that the active form of β-catenin was distinctly downregulated after DACT2 reexpression in both HCT116 and HT-29 cells (Figure 3a). Keeping with this finding, DACT2 overexpression inhibited SuperTOPflash reporter activities in both cell lines (Figure 3b). On the other hand, β-catenin-stimulated luciferase activity was significantly enhanced in DACT2 siRNA-transfected HEK-293 cells (Figure 3c). Besides, β-catenin nuclear translocation was notably decreased in DACT2-expressing HT-29 cells (Figure 3d). DACT2 led to relocation of β-catenin to cellular membrane rather than diffusely scattering in the cytoplasm (Figure 3d). It is generally known that HCT116 carries a gain-of-function mutation in β-catenin, which makes it resistant to degradation, whereas HT-29 has truncated adenomatous polyposis coli (APC), the key component of β-catenin destruction complex.10 Thus, inhibition of β-catenin signaling by DACT2 was not through promoting β-catenin degradation in these colon cancer cell lines. Accordingly, the total amount of β-catenin was unchanged (Figure 3a). To further confirm that inhibition of β-catenin signaling by DACT2 was independent of affecting β-catenin stability, SuperTOPflash luciferase reporter assay was performed. Reexpression of DACT2 significantly blocked the transcriptional activity of both wild-type β-catenin and constitutively active mutant β-catenin (S33Y), a degradation-resistant form in which serine 33 was substituted with tyrosine (Figure 3e). Wnt/β-catenin pathway is one of the important signaling pathways controlling epithelial–mesenchymal transition and driving cell metastasis.11,12 The disappearance of E-cadherin from the cellular membrane is considered as a crucial step in the progression of epithelial–mesenchymal transition.11,12 Thus, the effects of DACT2 on the expression and cellular localization of E-cadherin were examined. Our results showed that DACT2 upregulated the expression of E-cadherin (Figure 3a), which was enriched in cellular membrane, especially at cell–cell contact region (Figure 3f), indicating that DACT2 restored E-cadherin junction stability. On E-cadherin knockdown, cell invasive ability was significantly increased in DACT2-expressing HCT116 cells compared with control siRNA-transfected DACT2-expressing HCT116 cells (Figure 3g), indicating that E-cadherin blockade could partially relieve the anti-metastatic potential of DACT2.
DACT2 inhibits the transcriptional activity of β-catenin in the nucleus
The subcellular localization of DACT2 was examined in DACT2-expressing colon cancer cells. Immunofluorescent staining showed that DACT2 protein stayed predominantly in the cytoplasm (Figure 4a). Bioinformatic analysis indicated that DACT2 contains both putative nuclear export signal (NES) and nuclear localization signal (NLS), suggesting that DACT2 is probably a nucleocytoplasmic shuttling protein. To test this possibility, the distribution of DACT2 was examined by immunofluorescent staining after treatment with Leptomycin B, a potent and specific nuclear export inhibitor. Leptomycin B treatment induced strong nuclear accumulation of DACT2 (Figure 4b), implying that DACT2 actively shuttled between the nucleus and cytoplasm. This finding led us to speculate whether DACT2 in different subcelluar localization might have different roles in regulating Wnt/β-catenin signaling like APC.13,14 To identify NES and NLS motifs, DACT2 N-terminal region, middle region and C-terminal region were individually cloned (Figure 4c). Immunofluorescent staining demonstrated that DACT2 N-terminal region was completely localized in the cytoplasm and C-terminal region resided in the nucleus (Figure 4d), suggesting that NES and NLS were contained in the DACT2 N-terminal region and C-terminal region, respectively. DACT2 NES mutant was generated by replacing one critical hydrophobic residue (isoleucine 103) within NES with polar amino-acid serine, whereas DACT2 NLS was mutated by replacing one of the basic residues in lysine/arginine-rich motif in the C terminus with acidic glutamic acid (Figure 4e). Besides, DACT2 NES/NLS double mutant was also constructed. DACT2 NES mutant displayed a predominant nuclear distribution (Figure 4f), inferring that this mutation had totally impaired its nuclear export to the cytoplasm. In contrast, DACT2 NES/NLS double mutant showed a predominant cytoplasmic distribution (Figure 4f). Thus, NES/NLS double mutant was unable to stay in the nucleus as NLS mutation was sufficient for impairing its nuclear import function. Interestingly, both DACT2 NES and NLS mutants still exerted inhibitive effects on cell growth and β-catenin transcriptional activity (Figures 4g and h). Dapper family members could negatively regulate Wnt/β-catenin signaling by targeting Dvl for degradation,15,16 because Wnt-activated receptors need Dvl as a scaffold to recruit Axin and glycogen synthase kinase 3, resulting in disassembly of β-catenin destruction complex and consequently increased β-catenin stability.17 As this process happens in cytoplasm, inhibition of Wnt/β-catenin signaling by DACT2 NES mutant should not be through regulating Dvl. As expected, overexpression of wild-type DACT2 reduced Dvl2 expression, but DACT2 NES mutant did not (Figure 4i), indicating that DACT2 NES mutant might suppress β-catenin transcriptional activity in a more direct way in the nucleus. In addition, the expression level of E-cadherin was not significantly changed in DACT2 NES- and NLS mutant-expressing HCT116 cells compared with control cells (Figure 4j).
DACT2 suppressed Wnt/β-catenin signaling in the nucleus. (a) DACT2 stayed mainly in the cytoplasm. (b) Nuclear retention of DACT2 after Leptomycin B (LMB) treatment. HT-29 cells were transiently transfected with Flag-DACT2 construct followed by exposure to 50 nM LMB for 4 h, and DACT2 protein was analyzed by immunofluorescence staining. (c) Panel of DACT2 deletion constructs. (d) Celluar localization of DACT2 deletion variants. (e) Maps illustrating NES and NLS sequences within wild-type DACT2, and NES and NLS point mutation in DACT2 mutants. The NES sequence within DACT2 is a short amino-acid sequence consisting of four hydrophobic residues (leucine or isoleucine, highlighted in red) that targets it for export from nucleus to cytoplasm. DACT2 NES mutant harbored a point mutation within NES that caused an isoleucine-to-serine substitution in codon 103 (highlighted in blue). The NLS sequence is a lysine/arginine-rich motif in the C terminus that target DACT2 for import into the cell nucleus by nuclear transport. The point mutation of NLS caused a lysine-to-glutamic acid substitution in codon 758 (highlighted in blue). (f) NES mutation led to strong nuclear accumulation of DACT2 protein without LMB treatment. The DACT2 NES/NLS double mutant mainly stayed in cytoplasm, suggesting that NLS point mutation was sufficient for loss of importin-mediated nuclear localization. (g) Effects of DACT2 NES and NLS mutants on cell viability were evaluated by MTS assay in HCT116 cells. (h) Both DACT2 NES and NLS mutants negatively regulated Wnt/β-catenin signaling as evidenced by TOPflash reporter activity assay in HCT116 cells. DACT2 NES mutant inhibited TOPflash reporter activity induced by wild-type β-catenin as well as β-catenin mutant (S33Y) in HEK 293T cells. (i) Effect of DACT2 wild type and DACT2 NES mutant on the expression of Dvl2. HCT116 cells were transiently transfected with pcDNA3.1, pcDNA3.1-Flag-DACT2 or pcDNA3.1-Flag-DACT2-NES mutant. Forty-eight hours post transfection, cells were collected for western blot analysis. (j) DACT2 NES and NLS mutants did not significantly upregulate E-cadherin expression in HCT116 cells.
DACT2 directly interacts with β-catenin and disrupts β-catenin–lymphoid enhancer-binding factor 1 complex formation in the nucleus
To gain insight into the underlying mechanism of Wnt/β-catenin signaling inhibition by DACT2, we examined whether DACT2 physically interacted with the components of β-catenin–T-cell factor (TCF)/lymphoid enhancer-binding factor (LEF) transcriptional complex by co-immunoprecipitation. DACT2 was found to have a strong binding to β-catenin (Figure 5a). DACT2 NES and NLS mutants could also interact with β-catenin (Figure 5b). Moreover, DACT2 could also bind to TCF/LEF family member LEF1 (Figure 5c), which can form a transcriptional complex with β-catenin to activate Wnt target genes.18 We therefore examined the effect of DACT2 on the formation of β-catenin–LEF1 transcriptional complex. As shown in Figure 5d, a clear less-associated LEF1 was observed in β-catenin precipitates from DACT2-expressing cells, indicating that the interaction between LEF1 and β-catenin was evidently impaired in the presence of DACT2. Taken together, DACT2 binding to nuclear β-catenin, preventing it from forming complex with its partner LEF1, was an important mechanism for DACT2-mediated Wnt/β-catenin signaling inhibition in colon cancer.
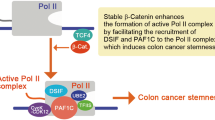
DACT2 directly disrupted β-catenin–LEF1 transcriptional complex formation. (a) Co-immunoprecipitation showed the physical interaction between DACT2 and β-catenin. Anti-Flag antibody was used to immunoprecipitate Flag-tagged DACT2 or β-catenin-containing complex from whole-cell extracts prepared from HCT116 cells co-expressing Flag-DACT2 and HA-β-catenin or Flag-β-catenin and GFP-DACT2. (b) Both DACT2 NES and NLS mutants interacted with β-catenin. (c) DACT2 interacted with LEF1. HCT116 cells were transfected with HA-LEF1 alone or together with Flag-DACT2 and were collected for anti-Flag immunoprecipitation. (d) The β-catenin–LEF1 complex formation was significantly impaired by DACT2 interaction. HCT116 cells were co-transfected with Flag-β-catenin and HA-LEF1-expressing constructs with or without Myc-DACT2 expression. The whole-cell lysates were subjected to immunoprecipitation using anti-Flag antibody and analyzed by immunoblotting. (e) DACT1 and DACT3 were frequently downregulated in colon cancer cell lines. (f) DACT1 and DACT3 had no interaction with β-catenin. (g) Proposed mechanistic models of tumor suppression by DACT2 in colon cancer.
DACT1 and DACT3 in colon cancer
Multiple sequence alignment showed that Dact gene family members share high sequence similarity within their N-terminal regions as well as C-terminal regions (Supplementary Figure). Bioinformatic analysis also found that both DACT1 and DACT3 have a typical NES in their N-terminal regions and at least one NLS in their C-terminal regions, suggesting that DACT1 and DACT3 are also nucleocytoplasmic shuttling proteins like DACT2. Semiquantitative reverse transcription PCR showed that DACT1 and DACT3 were downregulated in most of the colon cancer cell lines (Figure 5e). However, neither DACT1 nor DACT3 could interact with β-catenin as evidenced by co-immunoprecipitation assay (Figure 5f). DACT1 failed to regulate β-catenin transcriptional activity in HT29 cells (data not shown). On the basis of the previous findings, DACT3 inhibited colon cancer cell clonogenicity and promoted cell apoptosis by attenuating Wnt/β-catenin signaling pathway in colon cancer.16 Therefore, although both DACT2 and DACT3 were functional tumor suppressors through inhibiting Wnt/β-catenin signaling pathway, they probably acted on different molecular targets in Wnt/β-catenin signaling in colon cancer.
Promoter methylation of DACT2 is associated with poor survival of colon cancer patients
The clinical application of DACT2 methylation was evaluated in 67 primary colon cancers and in 12 healthy colon tissue samples. Partial and dense promoter methylation of DACT2 was detected in 43.3% (29/67) of cancer tissues, but none in 12 normal colon biopsies by BGS (Figure 6a). There was no correlation between the methylation status of DACT2 and clinicopathologic features (Supplementary Table 1). Whereas univariate Cox regression analysis showed that tumor-node-metastasis (TNM) stage was a significant prognostic factor (P<0.0005; Supplementary Table 2). After the adjustment for age, gender and TNM stage, DACT2 methylation in colon tumor tissues was found to predict poorer survival of colon cancer patients (hazard ratio (HR), 4.590; 95% confidence interval, 1.542–13.660; P=0.006; Table 1). Kaplan–Meier 5-year survival curves showed that colon cancer patients with DACT2 methylation had significantly shorter survival in early stage (I–III; P=0.009), but not for stage IV (Figure 6b).
DACT2 was frequently methylated in primary colon cancer. (a) Representative BGS results of DACT2 methylation status in colon cancer and normal colon tissue samples. (b) Kaplan–Meier survival curves of colon cancer patients, stratified by DACT2 methylation status, of early stage (TNM I–III) and late stage (TNM IV). In the early stage panel, survival was significantly shorter in the methylation group than others. In the stage IV panel, however, overall survival was not significantly different between the methylation and nonmethylation group.
Discussion
In this study, we found that DACT2 expression was frequently downregulated in colon cancer cell lines. The reduced expression was associated with promoter methylation as confirmed by direct BGS, inferring that DNA methylation is the main regulatory mechanism of DACT2 inactivation in colon cancer. Silencing of DACT2 might abolish tumor suppression so as to contribute to carcinogenesis. We tested the putative tumor suppressor function of DACT2 in colon cancer by both in vitro and in vivo assays. Reexpression of DACT2 in two silenced colon cancer cell lines (HCT116 and HT-29) showed significant growth inhibition by inhibiting cell proliferation and inducing cell apoptosis as evidenced by cell viability assay and Annexin V apoptosis assay. The diminution of tumor growth by DACT2 was further confirmed in nude mice in subcutaneous xenograft tumor model. On the other hand, siRNA-mediated knockdown of DACT2 in human embryonic kidney cell HEK-293 significantly increased clonogenicity. Moreover, DACT2 upregulated E-cadherin, restored epithelial junctional localization of E-cadherin/β-catenin complexes and reduced in vivo lung metastasis of colon cancer cells in nude mice. E-cadherin knockdown could partially relieve DACT2-mediated metastasis inhibition, inferring that E-cadherin was an essential target for DACT2 in regulating tumor cell invasion. We further elucidated the downstream signaling pathways that DACT2 exerted its tumor suppressor function in colon cancer and found that the anti-proliferative and anti-invasive effects derived by DACT2 were at least owing to inhibiting Wnt/β-catenin signaling, which is critical for the initiation and progression of most colon cancers.19 Collectively, through modulating Wnt/β-catenin signaling, DACT2 could function as a tumor suppressor gene by inhibiting tumor growth and metastasis.
DACT2 is a member of the dact family, which is first identified as a Dvl-interacting protein, and modulates the Wnt signalings by antagonizing Dvl.9 In the canonical Wnt pathway, as soon as Wnt ligand binds to a Frizzled receptor and low-density lipoprotein receptor-related proteins 5 and 6 (LRP5/6), Dvl is recruited by Frizzled receptor, then in turn promoting the recruitment of Axin and glycogen synthase kinase 3 to Wnt-activated receptors.17,20 This results in disassembly of β-catenin destruction complex and subsequently increased stability of β-catenin and its nuclear translocation. Therefore, targeting Dvl for degradation in cytoplasm is an upstream mechanism regulating β-catenin stability,21,22 which works only if the two conditions are met: first, Wnt signaling is activated under the stimulation of canonical Wnt ligands. Second, the destruction machinery, downstream of β-catenin and β-catenin itself, could function correctly without any mutations or other downstream interference. As we know, nearly all colon cancer cell lines including HCT116 and HT-29 have either loss-of-function mutation in destruction complex component APC or gain-of-function mutation in β-catenin, contributing to abnormal Wnt/β-catenin signaling activation.23 In our study, reexpression of DACT2 led to a significant downregulation of Wnt/β-catenin signaling in both HCT116 and HT-29 colon cancer cell lines without affecting β-catenin stability. Accordingly, DACT2 suppressed the TOPflash activity induced by degradation-resistant form of β-catenin (S33Y). Furthermore, nuclear-localized DACT2 NES mutant still inhibited β-catenin transcriptional activity, but lost its ability of regulating Dvl2 degradation, which happens in cytoplasm. Thus, although DACT2 could downregulate Dvl expression, the mechanism of Wnt/β-catenin signaling inhibition by DACT2 in colon cancer was independent of regulating Dvl.
The role of DACT2 in regulating Wnt/β-catenin signaling was further elucidated by the study of its subcelluar distribution. We found that DACT2 could dynamically shuttle into and out of nuclei, with its predominant steady-state localization in the cytoplasm dependent of its NES. Intriguingly, nuclear-localized DACT2 NES mutant retained its ability to suppress Wnt/β-catenin signaling, leading us to hypothesize that DACT2 might be directly participating in the process of β-catenin transcription in the nucleus. It is well established that TCF/LEF family is the main partner of β-catenin for transcriptional regulation of Wnt target genes.24 On Wnt stimulation, β-catenin is translocated into the nucleus and forms complex with TCF/LEF family member to displace its corepressors and recruit coactivators, hence switching on oncogenic Wnt target genes. In this regard, we tested the interaction between DACT2 and the components of β-catenin–TCF/LEF transcriptional complex. Co-immunoprecipitation results demonstrated that DACT2 strongly associated β-catenin as well as TCF/LEF family member LEF1, which was supported by earlier observations from Kivimäe et al.25 More importantly, β-catenin–LEF1 complex formation was greatly impaired by DACT2 interaction, indicating that DACT2 inhibited Wnt/β-catenin signaling by directly disrupting the formation of the β-catenin–LEF1 complex in the nucleus. As DACT2 interacted with both β-catenin and LEF1 and diminished their complex formation, DACT2 could not form a stable complex with β-catenin–LEF1, suggesting that DACT2 might compete with LEF1 for binding with β-catenin. The detailed molecular mechanism how DACT2 functions in disassembly of the β-catenin–LEF1 complex still needs to be addressed in the future study. Notably, APC, a key regulator of Wnt signaling, has been reported to modulate β-catenin activity in a similar way to DACT2.14 As a nucleocytoplasmic shuttling protein, APC not only degrades β-catenin by targeting it for ubiquitination in cytoplasm, but also sequesters β-catenin from its transcriptional partner TCF/LEF family in nucleus.14 Besides, DACT2 NLS mutant also suppressed Wnt/β-catenin signaling probably by preventing β-catenin nuclear translocation through direct interaction with β-catenin. Our findings extend our understanding of Wnt signaling regulation by elucidating the novel role for DACT2 in colon cancer. As DACT2 is constitutively expressed in normal colon tissue, we propose that DACT2 is an intrinsic regulator that tightly controls the basal β-catenin activity and expression level of Wnt target genes in normal colon epithelium.
In addition, reexpression of DACT2 upregulated E-cadherin and restored junctional localization of E-cadherin/β-catenin complexes at cellular membrane. Membrane-localized β-catenin interacts with E-cadherin to form adherens junctions, which is necessary for maintaining epithelial integrity in normal epithelium.26,27 β-catenin/E-cadherin complex at adherens junctions is also responsible for transmitting the contact inhibition signal that causes cells to stop proliferation once the epithelial sheet is complete,28,29 whereas the contact inhibition of proliferation is lost during tumor growth. Loss of E-cadherin/β-catenin at adherens junctions is emerging as a fundamental event in epithelial–mesenchymal transition, which results in loss of epithelial phenotype and disassembly of cell–cell junctions, hence reducing cell anchorage and increasing cell motility.26 Furthermore, β-catenin is released from E-cadherin at cell surface and translocated to the nucleus where it acts as a downstream oncogenic transcription factor. Nuclear β-catenin has been shown to activate the transcription of many epithelial–mesenchymal transition-related factors such as fibronectin and a variety of matrix metalloproteases, rendering them a more invasive phenotype.30 This process has been demonstrated to be closely associated with the progression and metastasis of many epithelial-derived tumors, especially colon cancer.26 Thus, reformation of β-catenin/E-cadherin-based adherens junctions was a critical mechanism by which DACT2 inhibited the invasive properties of colon cancer cells in vivo.
To ascertain the clinical application of DACT2 in colon cancer, we examined the promoter methylation of DACT2 in 67 primary colon cancers and 12 normal controls by BGS. DACT2 promoter methylation was observed in 43.3% of primary colon tumors, but none in normal colon tissues, suggesting that DACT2 inactivation caused by methylation is a frequent event in colon carcinogenesis. Loss of DACT2 expression by promoter methylation could lead to aberrant activation of Wnt/β-catenin signaling and ultimately tumorigenesis. DACT2 methylation detected in tumor tissues could significantly predict poorer survival in early stage colon cancer patients (Table 1, Figure 6b). Thus, DACT2 methylation could be regarded as a valuable new prognostic factor for patients with the early stage of colon cancer. The mechanism of how DACT2 methylation occurred in the progression of colon cancer is still unclear. The current findings suggest that in some cases, aberrant DNA methylation might be directly induced by preceding genetic or molecular alterations, while DNA methylation can also be altered by different environmental exposures, diet and chronic inflammation.31, 32, 33, 34, 35 The mechanism of how DACT2 was methylated in colon cancer needs to be addressed in epidemic analysis and animal models in future.
In conclusion, we have identified a novel functional tumor suppressor gene DACT2 inactivated by promoter methylation in colon cancer. DACT2 suppresses colon cancer growth through inhibiting Wnt/β-catenin signaling in two ways (Figure 5d). On one hand, DACT2 stringently controls β-catenin transcriptional activity by directly blocking β-catenin–LEF1 complex formation in the nucleus. On the other hand, DACT2 promotes β-catenin translocation to cytoplasm and restores the junctional localization of β-catenin/E-cadherin at adherens junctions via direct interaction with β-catenin. DACT2 methylation detected in tumor tissues was associated with shorter survival in the early stage of colon cancers. Thus, DACT2 methylation may serve as a useful biomarker for early stage colon cancer.
Materials and methods
Cell lines
Eight colon cancer cell lines (SW480, LoVo, LS 180, Caco-2, HT-29, DLD-1, SW620 and HCT116), HEK-293 and HEK 293T were obtained from the American Type Culture Collection (Manassas, VA, USA). Cell lines were maintained according to the protocols from American Type Culture Collection.
Primary colon tumor and normal tissue samples
Biopsy samples from primary colon tumor were obtained from colon cancer patients at the time of operation before any therapeutic intervention as described previously.36 A total of 67 patients with confirmed colon cancer were examined for DACT2 methylation. This included 36 men and 31 women. The median age of patients was 62.7 years (range, 32–84 years). In addition, 12 age-matched normal colon mucosae from healthy subjects were collected as normal control. The study protocol was approved by the Clinical Research Ethics Committee of the Chinese University of Hong Kong.
Plasmids
The information of the constructed plasmids is listed in Supplementary Table 3. pcDNA3.1-Flag-β-catenin, pcDNA3.1-Flag-β-catenin (S33Y), HA-tagged LEF1 and HA-tagged β-catenin constructs were kindly provided by Dr Zhijie Chang (School of Medicine, Tsinghua University).
RNA extraction and semiquantitative reverse transcription PCR
Total RNA was extracted using Trizol reagent (Invitrogen, Carlsbad, CA, USA). Complementary DNA was synthesized from total RNA using Transcriptor Reverse Transcriptase (Roche Applied Sciences, Indianapolis, IN, USA). DACT2 gene was amplified with β-actin as internal control. Primer sequences are listed in Supplementary Table 4.
Bisulfite modification of DNA and BGS
DNA bisulfite treatment and BGS analysis were performed as described previously (Supplementary Table 4; Figure 1b).37 Fifteen CpG sites spanning from −4 to +85 relative to the transcription start site were evaluated.
Establishment of stable DACT2-expressing cells
Retroviruses pBABE-puro-DACT2 or pBABE-puro control were produced by co-transfecting 293FT cells with pBABE-puro-Flag-DACT2 or pBABE-puro empty vector and two packaging plasmids pUMVC and pCMV-VSV-G (Addgene, Cambridge, MA, USA). HCT116 or HT-29 cells were infected with retrovirus and selected with puromycin (Invitrogen).
Cell viability assay
Cell viability was determined by the MTS assay (Promega, Madison, WI, USA).
Apoptosis assay
Apoptosis was determined by dual staining with APC Annexin V (Cat. No. 550474, BD Biosciences, San Jose, CA, USA) and 7-AAD (7-amino-actinomycin; Cat. No. 559925, BD Biosciences) as described previously.7
Colony formation assay
For DACT2 knockdown assay, HEK-293 cells were transfected with DACT2 siRNA (sc-95473, Santa Cruz Biotechnology, Dallas, TX, USA) or negative control siRNA (Santa Cruz Biotechnology). After 24 h transfection, 1000 cells were seeded and cultured for 10–14 days. Colonies (⩾50 cells/colony) were counted.
Inactivation of nuclear export by Leptomycin B
HT-29 cells were transfected with pcDNA3.1-Flag-DACT2. Twenty-four hours post transfection, Leptomycin B (Sigma-Aldrich, St Louis, MO, USA; dissolved in methanol) was added to the culture medium to a final concentration of 50 nM. After 4 h incubation, cells were then fixed with 4% paraformaldehyde and DACT2 cellular localization was analyzed by immunofluorescence staining.
Western blot
Total protein was extracted from cells' pellet. Thirty micrograms of protein from each sample were separated on 12% SDS-polyacrylamide gel electrophoresis and transferred onto nitrocellulose membranes (GE Healthcare, Piscataway, NJ, USA). Blots were immunostained with primary antibody and secondary antibody. GAPDH served as a loading control. Antibodies are listed in Supplementary Table 5.
Fluorescent immunohistochemistry
HCT116 or HT-29 cells were fixed and stained with primary antibodies (anti-FLAG M2, β-Catenin (D10A8) XP and E-Cadherin (24E10) (Cell Signaling Technology, Danvers, MA, USA): 1:50 dilution) as described previously.37 Images were captured by fluorescent microscopy.
Dual-luciferase reporter assay
HEK293T cells were plated in 24-well plates and co-transfected with various plasmids as indicated in the figures. Cells were colleceted 24 h post transfection and luciferase activities were analyzed by the dual-luciferase reporter assay system (Promega). Reporter activity was normalized to the control Renilla.
Invasion assay
Stable DACT2-expressing or control HCT116 cells were transfected with 50 nM E-cadherin siRNA (sense: 5'-GAUUGCACCGGUCGACAAA dTdT-3') (RiboBio, Guangzhou, China) or control siRNA (RiboBio). Forty-eight hours post transfection, cells were collected for western blot and Transwell assays using a BD BioCoat Growth Factor Reduced MATRIGEL Invasion Chamber (BD Biosciences) as described previously.38
Co-immunoprecipitation
Co-immunoprecipitation analyses were carried out as previously described.37 Briefly, total protein was extracted from HCT116 cells (~5 × 106/reaction) in radio-immunoprecipitation assay buffer. Immunoprecipitation was performed using anti-Flag M2 antibody (Sigma-Aldrich) and Protein G-agarose beads (sc-2002, Santa Cruz Biotechnology) overnight at 4 °C. Finally, the precipitated proteins were denatured and evaluated by western blot.
In vivo tumorigenicity and metastasis assays
Stable DACT2-expressing or control HCT116 cells (1 × 106 cells in 0.2 ml phosphate-buffered saline) were injected subcutaneously into the right or left dorsal flank of each 4-week-old male Balb/c nude mice, respectively. Tumor volume was measured every 2 days for 11 days. The tumors were excised and embedded in paraffin.
Male Balb/c nude mice (4 weeks old) were used, and each experimental group (control and DACT2) consisted of four mice. Stable DACT2-expressing or control HCT116 cells (1 × 106) were injected intravenously through the tail vein into each nude mouse. All mice were killed 8 weeks after injection. The lungs from each mouse were excised and embedded in paraffin for hematoxylin and eosin staining. All experimental procedures were approved by the Animal Ethics Committee of the Chinese University of Hong Kong.
Hematoxylin and eosin staining and immunohistochemistry
The paraffin-embedded tumor tissue and lung tissue sections were deparaffinized, rehydrated and stained with hematoxylin and eosin following standard protocols.39 Ki-67 immunohistochemistry was performed using the Histostain-Plus Bulk Kit (Invitrogen) as described previously.7
Statistical analysis
Data are presented as mean±s.d. The independent Student’s t-test was used to compare the difference between the two preselected groups. The difference in tumor growth rate between the two groups of mice was determined by repeated-measures analysis of variance. The χ2 test was used for comparison of patient characteristics and DACT2 methylation. The univariate and multivariate Cox regression analysis was performed to assess the prognostic value of DACT2 methylation. Overall survival in relation to methylation status was evaluated by the Kaplan–Meier 5-year survival curve and the log-rank test. Value of P<0.05 was considered to be statistically significant.
References
Parkin DM, Bray F, Ferlay J, Pisani P . Global cancer statistics, 2002. CA Cancer J Clin 2005; 55: 74–108.
Lao VV, Grady WM . Epigenetics and colorectal cancer. Nat Rev Gastroenterol Hepatol 2011; 8: 686–700.
Brott BK, Sokol SY . Frodo proteins: modulators of Wnt signaling in vertebrate development. Differentiation 2005; 73: 323–329.
Waxman JS, Hocking AM, Stoick CL, Moon RT . Zebrafish Dapper1 and Dapper2 play distinct roles in Wnt-mediated developmental processes. Development 2004; 131: 5909–5921.
Yuan G, Wang C, Ma C, Chen N, Tian Q, Zhang T et al. Oncogenic function of DACT1 in colon cancer through the regulation of β-catenin. PLoS ONE 2012; 7: e34004.
Suriben R, Kivimäe S, Fisher DA, Moon RT, Cheyette BN . Posterior malformations in Dact1 mutant mice arise through misregulated Vangl2 at the primitive streak. Nat Genet 2009; 41: 977–985.
Wang S, Kang W, Go MY, Tong JH, Li L, Zhang N et al. Dapper homolog 1 is a novel tumor suppressor in gastric cancer through inhibiting the nuclear factor-κB signaling pathway. Mol Med 2012; 18: 1402–1411.
Jia Y, Yang Y, Brock MV, Zhan Q, Herman JG, Guo M . Epigenetic regulation of DACT2, a key component of the Wnt signalling pathway in human lung cancer. J Pathol 2013; 230: 194–204.
Cheyette BN, Waxman JS, Miller JR, Takemaru K, Sheldahl LC, Khlebtsova N et al. Dapper, a Dishevelled-associated antagonist of beta-catenin and JNK signaling, is required for notochord formation. Dev Cell 2002; 2: 449–461.
Yang J, Zhang W, Evans PM, Chen X, He X, Liu C . Adenomatous polyposis coli (APC) differentially regulates beta-catenin phosphorylation and ubiquitination in colon cancer cells. J Biol Chem 2006; 281: 17751–17757.
Heuberger J, Birchmeier W . Interplay of cadherin-mediated cell adhesion and canonical Wnt signaling. Cold Spring Harb Perspect Biol 2010; 2: a002915.
Valenta T, Hausmann G, Basler K . The many faces and functions of β-catenin. EMBO J 2012; 31: 2714–2736.
Neufeld KL, Nix DA, Bogerd H, Kang Y, Beckerle MC, Cullen BR et al. Adenomatous polyposis coli protein contains two nuclear export signals and shuttles between the nucleus and cytoplasm. Proc Natl Acad Sci USA 2000; 97: 12085–12090.
Neufeld KL . Nuclear APC. Adv Exp Med Biol 2009; 656: 13–29.
Zhang L, Gao X, Wen J, Ning Y, Chen YG . Dapper 1 antagonizes Wnt signaling by promoting dishevelled degradation. J Biol Chem 2006; 281: 8607–8612.
Jiang X, Tan J, Li J, Kivimäe S, Yang X, Zhuang L et al. DACT3 is an epigenetic regulator of Wnt/beta-catenin signaling in colorectal cancer and is a therapeutic target of histone modifications. Cancer Cell 2008; 13: 529–541.
Gao C, Chen YG . Dishevelled: the hub of Wnt signaling. Cell Signal 2010; 22: 717–727.
Cadigan KM, Waterman ML . TCF/LEFs and Wnt signaling in the nucleus. Cold Spring Harb Perspect Biol 2012; 4: pii a007906.
Segditsas S, Tomlinson I . Colorectal cancer and genetic alterations in the Wnt pathway. Oncogene 2006; 25: 7531–7537.
Metcalfe C, Bienz M . Inhibition of GSK3 by Wnt signalling—two contrasting models. J Cell Sci 2011; 124: 3537–3544.
Angers S, Thorpe CJ, Biechele TL, Goldenberg SJ, Zheng N, MacCoss MJ et al. The KLHL12-Cullin-3 ubiquitin ligase negatively regulates the Wnt-beta-catenin pathway by targeting Dishevelled for degradation. Nat Cell Biol 2006; 8: 348–357.
Wei W, Li M, Wang J, Nie F, Li L . The E3 ubiquitin ligase ITCH negatively regulates canonical Wnt signaling by targeting dishevelled protein. Mol Cell Biol 2012; 32: 3903–3912.
Ikenoue T, Ijichi H, Kato N, Kanai F, Masaki T, Rengifo W et al. Analysis of the beta-catenin/T cell factor signaling pathway in 36 gastrointestinal and liver cancer cells. Jpn J Cancer Res 2002; 93: 1213–1220.
MacDonald BT, Tamai K, He X . Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev Cell 2009; 17: 9–26.
Kivimäe S, Yang XY, Cheyette BN . All Dact (Dapper/Frodo) scaffold proteins dimerize and exhibit conserved interactions with Vangl, Dvl, and serine/threonine kinases. BMC Biochem 2011; 12: 33.
Thiery JP, Sleeman JP . Complex networks orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell Biol 2006; 7: 131–142.
Kalluri R, Weinberg RA . The basics of epithelial-mesenchymal transition. J Clin Invest 2009; 119: 1420–1428.
Kim NG, Koh E, Chen X, Gumbiner BM . E-cadherin mediates contact inhibition of proliferation through Hippo signaling-pathway components. Proc Natl Acad Sci USA 2011; 108: 11930–11935.
Perrais M, Chen X, Perez-Moreno M, Gumbiner BM . E-cadherin homophilic ligation inhibits cell growth and epidermal growth factor receptor signaling independently of other cell interactions. Mol Biol Cell 2007; 18: 2013–2025.
Liu Y . New insights into epithelial-mesenchymal transition in kidney fibrosis. J Am Soc Nephrol 2010; 21: 212–222.
Brenner C, Deplus R, Didelot C, Loriot A, Viré E, De Smet C et al. Myc represses transcription through recruitment of DNA methyltransferase corepressor. EMBO J 2005; 24: 336–346.
Chiba T, Marusawa H, Ushijima T . Inflammation-associated cancer development in digestive organs: mechanisms and roles for genetic and epigenetic modulation. Gastroenterology 2012; 143: 550–563.
Choi SW, Friso S . Epigenetics: a new bridge between nutrition and health. Adv Nutr 2010; 1: 8–16.
Katsurano M, Niwa T, Yasui Y, Shigematsu Y, Yamashita S, Takeshima H et al. Early-stage formation of an epigenetic field defect in a mouse colitis model, and non-essential roles of T- and B-cells in DNA methylation induction. Oncogene 2012; 31: 342–351.
Hsieh CJ, Klump B, Holzmann K, Borchard F, Gregor M, Porschen R . Hypermethylation of the p16INK4a promoter in colectomy specimens of patients with long-standing and extensive ulcerative colitis. Cancer Res 1998; 58: 3942–3945.
Yu J, Ma X, Cheung KF, Li X, Tian L, Wang S et al. Epigenetic inactivation of T-box transcription factor 5, a novel tumor suppressor gene, is associated with colon cancer. Oncogene 2010; 29: 6464–6474.
Wang S, Cheng Y, Du W, Lu L, Zhou L, Wang H et al. Zinc-finger protein 545 is a novel tumour suppressor that acts by inhibiting ribosomal RNA transcription in gastric cancer. Gut 2013; 62: 833–841.
Ying J, Li H, Seng TJ, Langford C, Srivastava G, Tsao SW et al. Functional epigenetics identifies a protocadherin PCDH10 as a candidate tumor suppressor for nasopharyngeal, esophageal and multiple other carcinomas with frequent methylation. Oncogene 2006; 25: 1070–1080.
Yu J, Ip E, Dela Peña A, Hou JY, Sesha J, Pera N et al. COX-2 induction in mice with experimental nutritional steatohepatitis: role as pro-inflammatory mediator. Hepatology 2006; 43: 826–836.
Acknowledgements
This project was supported by Postdoctoral Special Foundation (2012M510317), National Natural Science Foundation of China (81301775), the Chinese Central Universities Funds (FRF-TP-12-172A), National Natural Science Foundation of China (81301775), 863 Program China (2012AA02A506), 973 Program China (2013CB531401) and Technology and Innovation Project Fund Shenzhen (JSGG20130412171021059).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Wang, S., Dong, Y., Zhang, Y. et al. DACT2 is a functional tumor suppressor through inhibiting Wnt/β-catenin pathway and associated with poor survival in colon cancer. Oncogene 34, 2575–2585 (2015). https://doi.org/10.1038/onc.2014.201
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2014.201
This article is cited by
-
Cellular signaling crosstalk between Wnt signaling and gap junctions inbenzo[a]pyrene toxicity
Cell Biology and Toxicology (2023)
-
Loss of Dact2 alleviates cisplatin-induced nephrotoxicity through regulation of the Igfl-MAPK pathway axis
Cell Biology and Toxicology (2023)
-
The new 6q27 tumor suppressor DACT2, frequently silenced by CpG methylation, sensitizes nasopharyngeal cancer cells to paclitaxel and 5-FU toxicity via β-catenin/Cdc25c signaling and G2/M arrest
Clinical Epigenetics (2018)
-
Prognostic DNA methylation markers for sporadic colorectal cancer: a systematic review
Clinical Epigenetics (2018)
-
Resetting the epigenetic balance of Polycomb and COMPASS function at enhancers for cancer therapy
Nature Medicine (2018)