Abstract
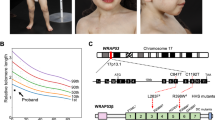
Polymorphisms and somatic mutations in Flap Endonuclease 1 (FEN1), an essential enzyme involved in DNA replication and repair, can lead to functional deficiencies of the FEN1 protein and a predisposition to cancer. We identified a FEN1 germline mutation that changed residue E359 to K in a patient whose family had a history of breast cancer. We determined that the E359K mutation, which is in the protein–protein domain of FEN1, abolished the interaction of FEN1 with Werner syndrome protein (WRN), an interaction that is critical for resolving stalled DNA replication forks. Furthermore, although the flap endonuclease activity of FEN1 E359K was unaffected, it failed to resolve bubble structures, which require the FEN1 gap-dependent endonuclease activity. To determine the etiological significance of E359K, we established a mouse model containing this mutation. E359K mouse embryonic fibroblasts (MEF) were more sensitive to DNA crosslinking agents that cause replication forks to stall. Cytological analysis suggested that the FEN1–WRN interaction was also required for telomere stability; mutant cell lines had fragile telomeres, increased numbers of spontaneous chromosomal anomalies and higher frequencies of transformation. Moreover, the incidence of cancer was significantly higher in mice homozygous for FEN1 E359K than in wild-type mice, suggesting that the FEN1 E359K mutation is oncogenic.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Branzei D, Foiani M . Maintaining genome stability at the replication fork. Nat Rev Mol Cell Biol 2010; 11: 208–219.
Gisselsson D, Pettersson L, Hoglund M, Heidenblad M, Gorunova L, Wiegant J et al. Chromosomal breakage-fusion-bridge events cause genetic intratumor heterogeneity. Proc Natl Acad Sci USA. 2000; 97: 5357–5362.
Lo AW, Sabatier L, Fouladi B, Pottier G, Ricoul M, Murnane JP et al. DNA amplification by breakage/fusion/bridge cycles initiated by spontaneous telomere loss in a human cancer cell line. Neoplasia 2002; 4: 531–538.
Nussenzweig A, Nussenzweig MC . Origin of chromosomal translocations in lymphoid cancer. Cell 2010; 141: 27–38.
Wang Y, Putnam CD, Kane MF, Zhang W, Edelmann L, Russell R et al. Mutation in Rpa1 results in defective DNA double-strand break repair, chromosomal instability and cancer in mice. Nat Genet 2005; 37: 750–755.
Bartkova J, Horejsi Z, Koed K, Kramer A, Tort F, Zieger K et al. DNA damage response as a candidate anti-cancer barrier in early human tumorigenesis. Nature 2005; 434: 864–870.
Bartkova J, Rezaei N, Liontos M, Karakaidos P, Kletsas D, Issaeva N et al. Oncogene-induced senescence is part of the tumorigenesis barrier imposed by DNA damage checkpoints. Nature 2006; 444: 633–637.
Bochman ML, Paeschke K, Zakian VA . DNA secondary structures: stability and function of G-quadruplex structures. Nat Rev Genet 2012; 13: 770–780.
Blackburn EH, Greider CW, Szostak JW . Telomeres and telomerase: the path from maize, Tetrahymena and yeast to human cancer and aging. Nat Med 2006; 12: 1133–1138.
de Lange T . Shelterin: the protein complex that shapes and safeguards human telomeres. Genes Dev 2005; 19: 2100–2110.
de Lange T . How shelterin solves the telomere end-protection problem. Cold Spring Harb Symp Quant Biol 2011; 75: 167–177.
Gilson E, Geli V . How telomeres are replicated. Nat Rev Mol Cell Biol 2007; 8: 825–838.
O'Sullivan RJ, Karlseder J . Telomeres: protecting chromosomes against genome instability. Nat Rev Mol Cell Biol 2010; 11: 171–181.
Michel B, Grompone G, Flores MJ, Bidnenko V . Multiple pathways process stalled replication forks. Proc Natl Acad Sci USA 2004; 101: 12783–12788, PMCID: 516472.
Zheng L, Jia J, Finger LD, Guo Z, Zer C, Shen B et al. Functional regulation of FEN1 nuclease and its link to cancer. Nucleic Acids Res 2011; 39: 781–794, PMCID: 3035468.
Freudenreich CH, Kantrow SM, Zakian VA . Expansion and length-dependent fragility of CTG repeats in yeast. Science 1998; 279: 853–856.
Guo Z, Chavez V, Singh P, Finger LD, Hang H, Hegde ML et al. Comprehensive mapping of the C-terminus of flap endonuclease-1 reveals distinct interaction sites for five proteins that represent different DNA replication and repair pathways. J Mol Biol 2008; 377: 679–690.
Lopes J, Ribeyre C, Nicolas A . Complex minisatellite rearrangements generated in the total or partial absence of Rad27/hFEN1 activity occur in a single generation and are Rad51 and Rad52 dependent. Mol Cell Biol 2006; 26: 6675–6689.
Singh P, Zheng L, Chavez V, Qiu J, Shen B . Concerted action of exonuclease and Gap-dependent endonuclease activities of FEN-1 contributes to the resolution of triplet repeat sequences (CTG)n- and (GAA)n-derived secondary structures formed during maturation of Okazaki fragments. J biol chem 2007; 282: 3465–3477.
Tishkoff DX, Filosi N, Gaida GM, Kolodner RD . A novel mutation avoidance mechanism dependent on S. cerevisiae RAD27 is distinct from DNA mismatch repair. Cell 1997; 88: 253–263.
Saharia A, Guittat L, Crockers S, Lim A, Steffen M, Kulkarni S et al. Flap endonuclease 1 contributes to telomere stability. Curr Biol 2008; 18: 496–500.
Saharia A, Teasley DC, Duxin JP, Dao B, Chiappinelli KB, Stewart SA . FEN1 ensures telomere stability by facilitating replication fork re-initiation. J Biol Chem 2010; 285: 27057–27066.
Sampathi S, Bhusari A, Shen B, Chai W . Human flap endonuclease I is in complex with telomerase and is required for telomerase-mediated telomere maintenance. J Biol Chem 2009; 284: 3682–3690, PMCID: 2635043.
Spiro C, Pelletier R, Rolfsmeier ML, Dixon MJ, Lahue RS, Gupta G et al. Inhibition of FEN-1 processing by DNA secondary structure at trinucleotide repeats. Mol Cell 1999; 4: 1079–1085.
Liu R, Qiu J, Finger LD, Zheng L, Shen B . The DNA-protein interaction modes of FEN-1 with gap substrates and their implication in preventing duplication mutations. Nucleic Acids Res 2006; 34: 1772–1784, PMCID: 1421507.
Zheng L, Zhou M, Chai Q, Parrish J, Xue D, Patrick SM et al. Novel function of the flap endonuclease 1 complex in processing stalled DNA replication forks. EMBO Rep 2005; 6: 83–89 PMCID: 1299223.
Kitano K, Kim SY, Hakoshima T . Structural basis for DNA strand separation by the unconventional winged-helix domain of RecQ helicase WRN. Structure 2010; 18: 177–187.
Sakurai S, Kitano K, Yamaguchi H, Hamada K, Okada K, Fukuda K et al. Structural basis for recruitment of human flap endonuclease 1 to PCNA. EMBO J 2005; 24: 683–693 PMCID: 549611.
Tsutakawa SE, Classen S, Chapados BR, Arvai AS, Finger LD, Guenther G et al. Human flap endonuclease structures, DNA double-base flipping, and a unified understanding of the FEN1 superfamily. Cell 2011; 145: 198–211 PMCID: 3086263.
Zheng L, Dai H, Qiu J, Huang Q, Shen B . Disruption of the FEN-1/PCNA interaction results in DNA replication defects, pulmonary hypoplasia, pancytopenia, and newborn lethality in mice. Mol Cell Biol 2007; 27: 3176–3186, PMCID: 1899923.
Bonner WM, Redon CE, Dickey JS, Nakamura AJ, Sedelnikova OA, Solier S et al. GammaH2AX and cancer. Nat Rev Cancer 2008; 8: 957–967, PMCID: 3094856.
Ganem NJ, Storchova Z, Pellman D . Tetraploidy, aneuploidy and cancer. Curr Opin Genet Dev 2007; 17: 157–162.
Ayyagari R, Gomes XV, Gordenin DA, Burgers PM . Okazaki fragment maturation in yeast. I. Distribution of functions between FEN1 AND DNA2. J Biol Chem 2003; 278: 1618–1625.
Li X, Li J, Harrington J, Lieber MR, Burgers PM . Lagging strand DNA synthesis at the eukaryotic replication fork involves binding and stimulation of FEN-1 by proliferating cell nuclear antigen. J Biol Chem 1995; 270: 22109–22112.
Zheng L, Shen B . Okazaki fragment maturation: nucleases take centre stage. J Mol Cell Biol 2011; 3: 23–30.
Zheng L, Dai H, Zhou M, Li M, Singh P, Qiu J et al. Fen1 mutations result in autoimmunity, chronic inflammation and cancers. Nat Med 2007; 13: 812–819.
Kucherlapati M, Yang K, Kuraguchi M, Zhao J, Lia M, Heyer J et al. Haploinsufficiency of Flap endonuclease (Fen1) leads to rapid tumor progression. Proc Natl Acad Sci USA 2002; 99: 9924–9929.
Larsen E, Gran C, Saether BE, Seeberg E, Klungland A . Proliferation failure and gamma radiation sensitivity of Fen1 null mutant mice at the blastocyst stage. Mol Cell Biol 2003; 23: 5346–5353.
Wu Z, Lin Y, Xu H, Dai H, Zhou M, Tsao S et al. High risk of benzo[alpha]pyrene-induced lung cancer in E160D FEN1 mutant mice. Mutat Res 2012; 731: 85–91, PMCID: 3268909.
Xu H, Zheng L, Dai H, Zhou M, Hua Y, Shen B et al. Chemical-induced cancer incidence and underlying mechanisms in Fen1 mutant mice. Oncogene, 30: 1072–1081.
Zheng L, Dai H, Zhou M, Li X, Liu C, Guo Z et al. Polyploid cells rewire DNA damage response networks to overcome replication stress-induced barriers for tumour progression. Nat Commun 2012; 3: 815.
Zheng L, Dai H, Hegde ML, Zhou M, Guo Z, Wu X et al. Fen1 mutations that specifically disrupt its interaction with PCNA cause aneuploidy-associated cancer. Cell Res 2011; 21: 1052–1067.
Bailey SM, Cornforth MN, Kurimasa A, Chen DJ, Goodwin EH . Strand-specific postreplicative processing of mammalian telomeres. Science 2001; 293: 2462–2465.
Murnane JP . Telomere loss as a mechanism for chromosome instability in human cancer. Cancer Res 2010; 70: 4255–4259, PMCID: 2967490.
Schvartzman JM, Sotillo R, Benezra R . Mitotic chromosomal instability and cancer: mouse modelling of the human disease. Nat Rev Cancer 2010; 10: 102–115.
Sweasy JB, Lang T, Starcevic D, Sun KW, Lai CC, Dimaio D et al. Expression of DNA polymerase {beta} cancer-associated variants in mouse cells results in cellular transformation. Proc Natl Acad Sci USA 2005; 102: 14350–14355, PMCID: 1242307.
Pierce BG, Hourai Y, Weng Z . Accelerating protein docking in ZDOCK using an advanced 3D convolution library. PLoS One 2011; 6: e24657, PMCID: 3176283.
Bernstein DA, Zittel MC, Keck JL . High-resolution structure of the E.coli RecQ helicase catalytic core. EMBO J 2003; 22: 4910–4921, PMCID: 204483.
Arnold K, Bordoli L, Kopp J, Schwede T . The SWISS-MODEL workspace: a web-based environment for protein structure homology modelling. Bioinformatics 2006; 22: 195–201.
Acknowledgements
We thank the City of Hope Pathology Core Facility for technical assistance with characterization of mouse cancer specimens (P30CA033572). We thank M Lee and B Armstrong in the City of Hope Microscopy Core Facility for assistance with characterization of chromosomal aberrations and D Stoppa-Lyonnet (Institut Curie) to provide the breast cancer patient DNA. This work was supported by NIH grant R01 CA073764 and Ligue Nationale contre le Cancer (EL2007/LNCC and EL2010/LNCC).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Chung, L., Onyango, D., Guo, Z. et al. The FEN1 E359K germline mutation disrupts the FEN1–WRN interaction and FEN1 GEN activity, causing aneuploidy-associated cancers. Oncogene 34, 902–911 (2015). https://doi.org/10.1038/onc.2014.19
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2014.19
This article is cited by
-
Flap endonuclease 1 and DNA-PKcs synergistically participate in stabilizing replication fork to encounter replication stress in glioma cells
Journal of Experimental & Clinical Cancer Research (2022)
-
Jianpi-yangwei decoction inhibits DNA damage repair in the drug resistance of gastric cancer by reducing FEN1 expression
BMC Complementary Medicine and Therapies (2020)
-
Control of structure-specific endonucleases to maintain genome stability
Nature Reviews Molecular Cell Biology (2017)
-
The FEN1 L209P mutation interferes with long-patch base excision repair and induces cellular transformation
Oncogene (2017)
-
Variants and haplotypes in Flap endonuclease 1 and risk of gallbladder cancer and gallstones: a population-based study in China
Scientific Reports (2015)