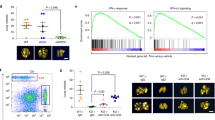
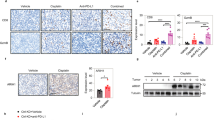
Abstract
The molecular basis for the resistance of tumor cells to cell-mediated cytotoxicity remains poorly understood and thus poses a major challenge for cancer immunotherapy. The present study was designed to determine whether the WNT1-inducible signaling pathway protein 2 (WISP2, also referred to as CCN5), a key regulator of tumor cell plasticity, interferes with tumor susceptibility to cytotoxic T-lymphocyte (CTL)-mediated lysis. We found that silencing WISP2 signaling in human breast adenocarcinoma MCF7 cells impairs CTL-mediated cell killing by a mechanism involving stem cell marker Kruppel-like factor-4 (KLF-4) induction and microRNA-7 (miR-7) downregulation. Inhibition of transforming growth factor beta (TGF-β) signaling using the A83-01 inhibitor in MCF7-shWISP2 cells resulted in a significant reversal of the epithelial-to-mesenchymal-transitioned (EMT) phenotype, the expression of KLF-4 and a partial recovery of target susceptibility to CTLs. More importantly, we showed that silencing KLF-4 was accompanied by a reduction in MCF7-shWISP2 resistance to CTLs. Using human breast cancer tissues, we demonstrated the coexpression of KLF-4 with EMT markers and TGF-β pathway signaling components. More importantly, we found that KLF-4 expression was accompanied by miR-7 inhibition, which is partly responsible for impairing CTL-mediated lysis. Thus, our data indicate that WISP2 has a role in regulating tumor cell susceptibility through EMT by inducing the TGF-β signaling pathway, KLF-4 expression and miR-7 inhibition. These studies indicate for the first time that WISP2 acts as an activator of CTL-induced killing and suggests that the loss of its function promotes evasion of immunosurveillance and the ensuing progression of the tumor.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol 2002; 3: 991–998.
Anichini A, Molla A, Mortarini R, Tragni G, Bersani I, Di Nicola M et al. An expanded peripheral T cell population to a cytotoxic T lymphocyte (Ctl)-defined, melanocyte-specific antigen in metastatic melanoma patients impacts on generation of peptide-specific ctls but does not overcome tumor escape from immune surveillance in metastatic lesions. J Exp Med 1999; 190: 651–667.
Chouaib S Integrating the quality of the cytotoxic response and tumor susceptibility into the design of protective vaccines in tumor immunotherapy. J Clin Invest 2003; 111: 595–597.
Hamai A, Benlalam H, Meslin F, Hasmim M, Carre T, Akalay I et al. Immune surveillance of human cancer: if the cytotoxic T-lymphocytes play the music, does the tumoral system call the tune? Tissue Antigens 2010; 75: 1–8.
Holzel M, Bovier A, Tuting T . Plasticity of tumour and immune cells: a source of heterogeneity and a cause for therapy resistance? Nat Rev Cancer 2013; 13: 365–376.
Yang J, Weinberg RA Epithelial-mesenchymal transition: at the crossroads of development and tumor metastasis. Dev Cell 2008; 14: 818–829.
Banerjee S, Saxena N, Sengupta K, Tawfik O, Mayo MS, Banerjee SK Wisp-2 gene in human breast cancer: estrogen and progesterone inducible expression and regulation of tumor cell proliferation. Neoplasia 2003; 5: 63–73.
Fritah A, Saucier C, De Wever O, Bracke M, Bieche I, Lidereau R et al. Role of Wisp-2/Ccn5 in the maintenance of a differentiated and noninvasive phenotype in human breast cancer cells. Mol Cell Biol 2008; 28: 1114–1123.
Banerjee S, Dhar G, Haque I, Kambhampati S, Mehta S, Sengupta K et al. Ccn5/Wisp-2 expression in breast adenocarcinoma is associated with less frequent progression of the disease and suppresses the invasive phenotypes of tumor cells. Cancer Res 2008; 68: 7606–7612.
Sabbah M, Prunier C, Ferrand N, Megalophonos V, Lambein K, De Wever O et al. Ccn5, a novel transcriptional repressor of the transforming growth factor beta signaling pathway. Mol Cell Biol 2011; 31: 1459–1469.
Dhar G, Banerjee S, Dhar K, Tawfik O, Mayo MS, Vanveldhuizen PJ et al. Gain of oncogenic function of P53 mutants induces invasive phenotypes in human breast cancer cells by silencing Ccn5/Wisp-2. Cancer Res 2008; 68: 4580–4587.
Frewer KA, Sanders AJ, Owen S, Frewer NC, Hargest R, Jiang WG A role for Wisp2 in colorectal cancer cell invasion and motility. Cancer Genomics Proteomics 2013; 10: 187–196.
Akalay I, Janji B, Hasmim M, Noman MZ, Andre F, De Cremoux P et al. Epithelial-to-mesenchymal transition and autophagy induction in breast carcinoma promote escape from T-cell-mediated lysis. Cancer Res 2013; 73: 2418–2427.
Akalay I, Janji B, Hasmim M, Noman MZ, Thiery JP, Mami-Chouaib F et al. EMT impairs breast carcinoma cell susceptibility to ctl-mediated lysis through autophagy induction. Autophagy 2013; 9: 1104–1106.
Verhaak RG, Hoadley KA, Purdom E, Wang V, Qi Y, Wilkerson MD et al. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in Pdgfra, Idh1, Egfr, and Nf1. Cancer Cell 2010; 17: 98–110.
Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA 2005; 102: 15545–15550.
Neve RM, Chin K, Fridlyand J, Yeh J, Baehner FL, Fevr T et al. A collection of breast cancer cell lines for the study of functionally distinct cancer subtypes. Cancer Cell 2006; 10: 515–527.
Dorothee G, Echchakir H, Le Maux Chansac B, Vergnon I, El Hage F, Moretta A et al. Functional and molecular characterization of a Kir3dl2/P140 expressing tumor-specific cytotoxic T lymphocyte clone infiltrating a human lung carcinoma. Oncogene 2003; 22: 7192–7198.
Hasmim M, Noman MZ, Lauriol J, Benlalam H, Mallavialle A, Rosselli F et al. Hypoxia-dependent inhibition of tumor cell susceptibility to Ctl-mediated lysis involves nanog induction in target cells. J Immunol 2011; 187: 4031–4039.
Li MO, Wan YY, Sanjabi S, Robertson AK, Flavell RA Transforming growth factor-beta regulation of immune responses. Annu Rev Immunol 2006; 24: 99–146.
Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell 2008; 133: 704–715.
Liu Z, Bandyopadhyay A, Nichols RW, Wang L, Hinck AP, Wang S et al. Blockade of autocrine Tgf-beta signaling inhibits stem cell phenotype, survival, and metastasis of murine breast cancer cells. J Stem Cell Res Ther 2012; 2: 1–8.
Reiman JM, Knutson KL, Radisky DC Immune promotion of epithelial-mesenchymal transition and generation of breast cancer stem cells. Cancer Res 2010; 70: 3005–3008.
Okuda H, Xing F, Pandey PR, Sharma S, Watabe M, Pai SK et al. Mir-7 suppresses brain metastasis of breast cancer stem-like cells by modulating Klf4. Cancer Res 2013; 73: 1434–1444.
Rebucci M, Michiels C Molecular aspects of cancer cell resistance to chemotherapy. Biochem Pharmacol 2013; 85: 1219–1226.
Shekhani MT, Jayanthy AS, Maddodi N, Setaluri V Cancer stem cells and tumor transdifferentiation: implications for novel therapeutic strategies. Am J Stem Cells 2013; 2: 52–61.
Hu D, Wan Y Regulation of Kruppel-like factor 4 by the anaphase promoting complex pathway is involved in Tgf-beta signaling. J Biol Chem 2011; 286: 6890–6901.
Liu YN, Abou-Kheir W, Yin JJ, Fang L, Hynes P, Casey O et al. Critical and reciprocal regulation of Klf4 and slug in transforming growth factor beta-initiated prostate cancer epithelial-mesenchymal transition. Mol Cell Biol 2012; 32: 941–953.
Zhang XH, Zheng B, Gu C, Fu JR, Wen JK . TGF-beta1 downregulates AT1 receptor expression via PKC-delta-mediated Sp1 dissociation from KLF4 and Smad-mediated PPAR-gamma association with KLF4. Arterioscler Thromb Vasc Biol 2012; 32: 1015–1023.
Yang Y, Goldstein BG, Chao HH, Katz JP . Klf4 and Klf5 regulate proliferation, apoptosis and invasion in esophageal cancer cells. Cancer Biol Ther 2005; 4: 1216–1221.
El-Karim EA, Hagos EG, Ghaleb AM, Yu B, Yang VW Kruppel-like factor 4 regulates genetic stability in mouse embryonic fibroblasts. Mol Cancer 2013; 12: 89.
Mohammad KS, Javelaud D, Fournier PG, Niewolna M, McKenna CR, Peng XH et al. Tgf-Beta-Ri kinase inhibitor Sd-208 reduces the development and progression of melanoma bone metastases. Cancer Res 2011; 71: 175–184.
Xu N, Papagiannakopoulos T, Pan G, Thomson JA, Kosik KS Microrna-145 regulates Oct4, Sox2, and Klf4 and represses pluripotency in human embryonic stem cells. Cell 2009; 137: 647–658.
Giles KM, Brown RA, Epis MR, Kalinowski FC, Leedman PJ Mirna-7-5p inhibits melanoma cell migration and invasion. Biochem Biophys Res Commun 2013; 430: 706–710.
Fang Y, Xue JL, Shen Q, Chen J, Tian L Microrna-7 inhibits tumor growth and metastasis by targeting the phosphoinositide 3-kinase/Akt pathway in hepatocellular carcinoma. Hepatology 2012; 55: 1852–1862.
Johnson WE, Li C, Rabinovic A . Adjusting batch effects in microarray expression data using empirical bayes methods. Biostatistics 2007; 8: 118–127.
Gatza ML, Lucas JE, Barry WT, Kim JW, Wang Q, Crawford MD et al. A pathway-based classification of human breast cancer. Proc Natl Acad Sci USA 2010; 107: 6994–6999.
El Hage F, Stroobant V, Vergnon I, Baurain JF, Echchakir H, Lazar V et al. Preprocalcitonin signal peptide generates a cytotoxic T lymphocyte-defined tumor epitope processed by a proteasome-independent pathway. Proc Natl Acad Sci USA 2008; 105: 10119–10124.
Magnon C, Opolon P, Ricard M, Connault E, Ardouin P, Galaup A et al. Radiation and inhibition of angiogenesis by canstatin synergize to induce Hif-1alpha-mediated tumor apoptotic switch. J Clin Invest 2007; 117: 1844–1855.
Acknowledgements
This work was supported by grants from INSERM, la Ligue Nationale Contre le Cancer, l’Association de Recherche sur le Cancer, A*STAR Institute of Molecular Cell Biology, Cancer Science Institute National University of Singapore core grants, CRP-Santé and Fondation Cancer, Luxembourg.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Rights and permissions
About this article
Cite this article
Akalay, I., Tan, T., Kumar, P. et al. Targeting WNT1-inducible signaling pathway protein 2 alters human breast cancer cell susceptibility to specific lysis through regulation of KLF-4 and miR-7 expression. Oncogene 34, 2261–2271 (2015). https://doi.org/10.1038/onc.2014.151
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2014.151
This article is cited by
-
The loss of B7-H4 expression in breast cancer cells escaping from T cell cytotoxicity contributes to epithelial-to-mesenchymal transition
Breast Cancer Research (2023)
-
Harnessing epithelial-mesenchymal plasticity to boost cancer immunotherapy
Cellular & Molecular Immunology (2023)
-
EMT-induced immune evasion: connecting the dots from mechanisms to therapy
Clinical and Experimental Medicine (2023)
-
Differential expression profile of plasma exosomal microRNAs in acute type A aortic dissection with acute lung injury
Scientific Reports (2022)
-
Identification of the prognostic value of a 2-gene signature of the WNT gene family in UCEC using bioinformatics and real-world data
Cancer Cell International (2021)