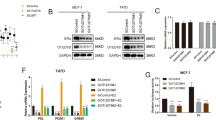
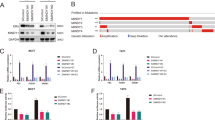
Abstract
Estrogen receptor-alpha (ERα) is an important biomarker used to classify and direct therapy decisions in breast cancer (BC). Both ERα protein and its transcript, ESR1, are used to predict response to tamoxifen therapy, yet certain tumors have discordant levels of ERα protein and ESR1, which is currently unexplained. Cellular ERα protein levels can be controlled post-translationally by the ubiquitin-proteasome pathway through a mechanism that depends on phosphorylation at residue S118. Phospho-S118 (pS118-ERα) is a substrate for the peptidyl prolyl isomerase, Pin1, which mediates cis-trans isomerization of the pS118-P119 bond to enhance ERα transcriptional function. Here, we demonstrate that Pin1 can increase ERα protein without affecting ESR1 transcript levels by inhibiting proteasome-dependent receptor degradation. Pin1 disrupts ERα ubiquitination by interfering with receptor interactions with the E3 ligase, E6AP, which also is shown to bind pS118-ERα. Quantitative in situ assessments of ERα protein, ESR1, and Pin1 in human tumors from a retrospective cohort show that Pin1 levels correlate with ERα protein but not to ESR1 levels. These data show that ERα protein is post-translationally regulated by Pin1 in a proportion of breast carcinomas. As Pin1 impacts both ERα protein levels and transactivation function, these data implicate Pin1 as a potential surrogate marker for predicting outcome of ERα-positive BC.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Dowsett M, Cuzick J, Ingle J, Coates A, Forbes J, Bliss J et al. Meta-analysis of breast cancer outcomes in adjuvant trials of aromatase inhibitors versus tamoxifen. J Clin Oncol 2010; 28: 509–518.
Early Breast Cancer Trialists' Collaborative Group EBCTCG, Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. The Lancet 2005; 365: 1687–1717.
Harrell JC, Dye WW, Allred DC, Jedlicka P, Spoelstra NS, Sartorius CA et al. Estrogen receptor positive breast cancer metastasis: altered hormonal sensitivity and tumor aggressiveness in lymphatic vessels and lymph nodes. Cancer Res 2006; 66: 9308–9315.
Harrell JC, Dye WW, Harvell DM, Pinto M, Jedlicka P, Sartorius CA et al. Estrogen insensitivity in a model of estrogen receptor positive breast cancer lymph node metastasis. Cancer Res 2007; 67: 10582–10591.
Khan SA, Rogers MA, Khurana KK, Meguid MM, Numann PJ . Estrogen receptor expression in benign breast epithelium and breast cancer risk. J Natl Cancer Inst 1998; 90: 37–42.
Frech MS, Halama ED, Tilli MT, Singh B, Gunther EJ, Chodosh LA et al. Deregulated estrogen receptor alpha expression in mammary epithelial cells of transgenic mice results in the development of ductal carcinoma in situ. Cancer Res 2005; 65: 681–685.
Fowler AM, Solodin N, Preisler-Mashek MT, Zhang P, Lee AV, Alarid ET . Increases in estrogen receptor-alpha concentration in breast cancer cells promote serine 118/104/106-independent AF-1 transactivation and growth in the absence of estrogen. FASEB J 2004; 18: 81–93.
Fowler AM, Solodin NM, Valley CC, Alarid ET . Altered target gene regulation controlled by estrogen receptor-alpha concentration. Mol Endocrinol 2006; 20: 291–301.
Bordeaux JM, Cheng H, Welsh AW, Haffty BG, Lannin DR, Wu X et al. Quantitative in situ measurement of estrogen receptor mRNA predicts response to tamoxifen. PLoS One 2012; 7: e36559.
Gong Y, Yan K, Lin F, Anderson K, Sotiriou C, Andre F et al. Determination of oestrogen-receptor status and ERBB2 status of breast carcinoma: a gene-expression profiling study. Lancet Oncol 2007; 8: 203–211.
Tora L, White J, Brou C, Tasset D, Webster N, Scheer E et al. The human estrogen receptor has two independent nonacidic transcriptional activation functions. Cell 1989; 59: 477–487.
Valley CC, Metivier R, Solodin NM, Fowler AM, Mashek MT, Hill L et al. Differential regulation of estrogen-inducible proteolysis and transcription by the estrogen receptor alpha N terminus. Mol Cell Biol 2005; 25: 5417–5428.
Lu KP, Finn G, Lee TH, Nicholson LK . Prolyl cis-trans isomerization as a molecular timer. Nat Chem Biol 2007; 3: 619–629.
Lu KP, Liou YC, Zhou XZ . Pinning down proline-directed phosphorylation signaling. Trends Cell Biol 2002; 12: 164–172.
Lu KP, Zhou XZ . The prolyl isomerase PIN1: a pivotal new twist in phosphorylation signalling and disease. Nat Rev Mol Cell Biol 2007; 8: 904–916.
Wulf G, Finn G, Suizu F, Lu KP . Phosphorylation-specific prolyl isomerization: is there an underlying theme? Nat Cell Biol 2005; 7: 435–441.
Ranganathan R, Lu KP, Hunter T, Noel JP . Structural and functional analysis of the mitotic rotamase Pin1 suggests substrate recognition is phosphorylation dependent. Cell 1997; 89: 875–886.
Yaffe MB, Schutkowski M, Shen M, Zhou XZ, Stukenberg PT, Rahfeld JU et al. Sequence-specific and phosphorylation-dependent proline isomerization: a potential mitotic regulatory mechanism. Science 1997; 278: 1957–1960.
Rajbhandari P, Finn G, Solodin NM, Singarapu KK, Sahu SC, Markley JL et al. Regulation of estrogen receptor alpha N-terminus conformation and function by peptidyl prolyl isomerase Pin1. Mol Cell Biol 2012; 32: 445–457.
Wulf G, Ryo A, Liou YC, Lu KP . The prolyl isomerase Pin1 in breast development and cancer. Breast Cancer Res 2003; 5: 76–82.
Wulf GM, Ryo A, Wulf GG, Lee SW, Niu T, Petkova V et al. Pin1 is overexpressed in breast cancer and cooperates with Ras signaling in increasing the transcriptional activity of c-Jun towards cyclin D1. Embo J 2001; 20: 3459–3472.
Wulf G, Garg P, Liou YC, Iglehart D, Lu KP . Modeling breast cancer in vivo and ex vivo reveals an essential role of Pin1 in tumorigenesis. Embo J 2004; 23: 3397–3407.
Zhou XZ, Kops O, Werner A, Lu PJ, Shen M, Stoller G et al. Pin1-dependent prolyl isomerization regulates dephosphorylation of Cdc25C and tau proteins. Mol Cell 2000; 6: 873–883.
Bhatt S, Xiao Z, Meng Z, Katzenellenbogen BS . Phosphorylation by p38 mitogen-activated protein kinase promotes estrogen receptor alpha turnover and functional activity via the SCF(Skp2) proteasomal complex. Mol Cell Biol 2012; 32: 1928–1943.
Eakin CM, Maccoss MJ, Finney GL, Klevit RE . Estrogen receptor alpha is a putative substrate for the BRCA1 ubiquitin ligase. Proc Natl Acad Sci USA 2007; 104: 5794–5799.
Li L, Li Z, Howley PM, Sacks DB . E6AP and calmodulin reciprocally regulate estrogen receptor stability. J Biol Chem 2006; 281: 1978–1985.
Nakajima A, Maruyama S, Bohgaki M, Miyajima N, Tsukiyama T, Sakuragi N et al. Ligand-dependent transcription of estrogen receptor alpha is mediated by the ubiquitin ligase EFP. Biochem Biophys Res Commun 2007; 357: 245–251.
Tateishi Y, Kawabe Y, Chiba T, Murata S, Ichikawa K, Murayama A et al. Ligand-dependent switching of ubiquitin-proteasome pathways for estrogen receptor. Embo J 2004; 23: 4813–4823.
Duong V, Boulle N, Daujat S, Chauvet J, Bonnet S, Neel H et al. Differential regulation of estrogen receptor alpha turnover and transactivation by Mdm2 and stress-inducing agents. Cancer Res 2007; 67: 5513–5521.
Sun J, Zhou W, Kaliappan K, Nawaz Z, Slingerland JM . ERalpha phosphorylation at Y537 by Src triggers E6-AP-ERalpha binding, ERalpha ubiquitylation, promoter occupancy, and target gene expression. Mol Endocrinol 2012; 26: 1567–1577.
Ramamoorthy S, Nawaz Z . E6-associated protein (E6-AP) is a dual function coactivator of steroid hormone receptors. Nucl Recept Signal 2008; 6: e006.
Hennig L, Christner C, Kipping M, Schelbert B, Rucknagel KP, Grabley S et al. Selective inactivation of parvulin-like peptidyl-prolyl cis/trans isomerases by juglone. Biochemistry 1998; 37: 5953–5960.
Min SH, Lau AW, Lee TH, Inuzuka H, Wei S, Huang P et al. Negative regulation of the stability and tumor suppressor function of Fbw7 by the Pin1 prolyl isomerase. Mol Cell 2012; 46: 771–783.
Zhou W, Yang Q, Low CB, Karthik BC, Wang Y, Ryo A et al. Pin1 catalyzes conformational changes of Thr-187 in p27Kip1 and mediates its stability through a polyubiquitination process. J Biol Chem 2009; 284: 23980–23988.
Fujimoto Y, Shiraki T, Horiuchi Y, Waku T, Shigenaga A, Otaka A et al. Proline cis/trans-isomerase Pin1 regulates peroxisome proliferator-activated receptor gamma activity through the direct binding to the activation function-1 domain. J Biol Chem 2010; 285: 3126–3132.
Cheng J, Zhang C, Shapiro DJ . A functional serine 118 phosphorylation site in estrogen receptor-alpha is required for down-regulation of gene expression by 17beta-estradiol and 4-hydroxytamoxifen. Endocrinology 2007; 148: 4634–4641.
Duplessis TT, Williams CC, Hill SM, Rowan BG . Phosphorylation of Estrogen Receptor alpha at serine 118 directs recruitment of promoter complexes and gene-specific transcription. Endocrinology 2011; 152: 2517–2526.
Lonard DM, Nawaz Z, Smith CL, O’Malley BW . The 26S proteasome is required for estrogen receptor-alpha and coactivator turnover and for efficient estrogen receptor-alpha transactivation. Mol Cell 2000; 5: 939–948.
Reid G, Hubner MR, Metivier R, Brand H, Denger S, Manu D et al. Cyclic, proteasome-mediated turnover of unliganded and liganded ERalpha on responsive promoters is an integral feature of estrogen signaling. Mol Cell 2003; 11: 695–707.
Muratani M, Tansey WP . How the ubiquitin-proteasome system controls transcription. Nat Rev Mol Cell Biol 2003; 4: 192–201.
Wang Y, Zong H, Chi Y, Hong Y, Yang Y, Zou W et al. Repression of estrogen receptor alpha by CDK11p58 through promoting its ubiquitin-proteasome degradation. J Biochem 2009; 145: 331–343.
Wei X, Xu H, Kufe D . MUC1 oncoprotein stabilizes and activates estrogen receptor alpha. Mol Cell 2006; 21: 295–305.
Borras M, Hardy L, Lempereur F, el Khissiin AH, Legros N, Gol-Winkler R et al. Estradiol-induced down-regulation of estrogen receptor. Effect of various modulators of protein synthesis and expression. J Steroid Biochem Mol Biol 1994; 48: 325–336.
Lu Q, Surks HK, Ebling H, Baur WE, Brown D, Pallas DC et al. Regulation of estrogen receptor alpha-mediated transcription by a direct interaction with protein phosphatase 2A. J Biol Chem 2003; 278: 4639–4645.
Marsaud V, Gougelet A, Maillard S, Renoir JM . Various phosphorylation pathways, depending on agonist and antagonist binding to endogenous estrogen receptor alpha (ERalpha), differentially affect ERalpha extractability, proteasome-mediated stability, and transcriptional activity in human breast cancer cells. Mol Endocrinol 2003; 17: 2013–2027.
Kato K, Ueoka Y, Hachiya T, Nishida J, Wake N . Contribution of enhanced transcriptional activation by ER to [12Val] K-Ras mediated NIH3T3 cell transformation. Oncogene 1997; 15: 3037–3046.
Atsriku C, Britton DJ, Held JM, Schilling B, Scott GK, Gibson BW et al. Systematic mapping of posttranslational modifications in human estrogen receptor-alpha with emphasis on novel phosphorylation sites. Mol Cell Proteomics 2009; 8: 467–480.
Khanal P, Yun HJ, Lim SC, Ahn SG, Yoon HE, Kang KW et al. Proyl isomerase Pin1 facilitates ubiquitin-mediated degradation of cyclin-dependent kinase 10 to induce tamoxifen resistance in breast cancer cells. Oncogene 2012; 31: 3845–3856.
Namgoong GM, Khanal P, Cho HG, Lim SC, Oh YK, Kang BS et al. The prolyl-isomerase pin1 induces LC-3 expression and mediates tamoxifen resistance in breast cancer. J Biol Chem 2010; 285: 23829–23841.
Stanya KJ, Liu Y, Means AR, Kao HY . Cdk2 and Pin1 negatively regulate the transcriptional corepressor SMRT. J Cell Biol 2008; 183: 49–61.
Alarid ET . Lives and times of nuclear receptors. Mol Endocrinol 2006; 20: 1972–1981.
Faus H, Haendler B . Post-translational modifications of steroid receptors. Biomed Pharmacother 2006; 60: 520–528.
Rochette-Egly C . Nuclear receptors: integration of multiple signalling pathways through phosphorylation. Cell Signal 2003; 15: 355–366.
Chen HZ, Li L, Wang WJ, Du XD, Wen Q, He JP et al. Prolyl isomerase Pin1 stabilizes and activates orphan nuclear receptor TR3 to promote mitogenesis. Oncogene 2012; 31: 2876–2887.
Mantovani F, Piazza S, Gostissa M, Strano S, Zacchi P, Mantovani R et al. Pin1 links the activities of c-Abl and p300 in regulating p73 function. Mol Cell 2004; 14: 625–636.
Pulikkan JA, Dengler V, Peer Zada AA, Kawasaki A, Geletu M, Pasalic Z et al. Elevated PIN1 expression by C/EBPalpha-p30 blocks C/EBPalpha-induced granulocytic differentiation through c-Jun in AML. Leukemia 2010; 24: 914–923.
Ryo A, Nakamura M, Wulf G, Liou YC, Lu KP . Pin1 regulates turnover and subcellular localization of beta-catenin by inhibiting its interaction with APC. Nat Cell Biol 2001; 3: 793–801.
Ryo A, Suizu F, Yoshida Y, Perrem K, Liou YC, Wulf G et al. Regulation of NF-kappaB signaling by Pin1-dependent prolyl isomerization and ubiquitin-mediated proteolysis of p65/RelA. Mol Cell 2003; 12: 1413–1426.
Wulf GM, Liou YC, Ryo A, Lee SW, Lu KP . Role of Pin1 in the regulation of p53 stability and p21 transactivation, and cell cycle checkpoints in response to DNA damage. J Biol Chem 2002; 277: 47976–47979.
Gao X, Mohsin SK, Gatalica Z, Fu G, Sharma P, Nawaz Z . Decreased expression of e6-associated protein in breast and prostate carcinomas. Endocrinology 2005; 146: 1707–1712.
Ramamoorthy S, Tufail R, Hokayem JE, Jorda M, Zhao W, Reis Z et al. Overexpression of ligase defective E6-associated protein, E6-AP, results in mammary tumorigenesis. Breast Cancer Res Treat 2012; 132: 97–108.
Picard N, Charbonneau C, Sanchez M, Licznar A, Busson M, Lazennec G et al. Phosphorylation of activation function-1 regulates proteasome-dependent nuclear mobility and E6-associated protein ubiquitin ligase recruitment to the estrogen receptor beta. Mol Endocrinol 2008; 22: 317–330.
Lannigan DA . Estrogen receptor phosphorylation. Steroids 2003; 68: 1–9.
Kim C, Tang G, Pogue-Geile KL, Costantino JP, Baehner FL, Baker J et al. Estrogen receptor (ESR1) mRNA expression and benefit from tamoxifen in the treatment and prevention of estrogen receptor-positive breast cancer. J Clin Oncol 2011; 29: 4160–4167.
Kumar V, Green S, Stack G, Berry M, Jin JR, Chambon P . Functional domains of the human estrogen receptor. Cell 1987; 51: 941–951.
Zheng H, You H, Zhou XZ, Murray SA, Uchida T, Wulf G et al. The prolyl isomerase Pin1 is a regulator of p53 in genotoxic response. Nature 2002; 419: 849–853.
Liou YC, Ryo A, Huang HK, Lu PJ, Bronson R, Fujimori F et al. Loss of Pin1 function in the mouse causes phenotypes resembling cyclin D1-null phenotypes. Proc Natl Acad Sci USA 2002; 99: 1335–1340.
Lu PJ, Wulf G, Zhou XZ, Davies P, Lu KP . The prolyl isomerase Pin1 restores the function of Alzheimer-associated phosphorylated tau protein. Nature 1999; 399: 784–788.
Ellison-Zelski SJ, Solodin NM, Alarid ET . Repression of ESR1 through actions of estrogen receptor alpha and Sin3A at the proximal promoter. Mol Cell Biol 2009; 29: 4949–4958.
Livak KJ, Schmittgen TD . Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001; 25: 402–408.
Welsh AW, Moeder CB, Kumar S, Gershkovich P, Alarid ET, Harigopal M et al. Standardization of estrogen receptor measurement in breast cancer suggests false-negative results are a function of threshold intensity rather than percentage of positive cells. J Clin Oncol 2011; 29: 2978–2984.
Camp RL, Chung GG, Rimm DL . Automated subcellular localization and quantification of protein expression in tissue microarrays. Nat Med 2002; 8: 1323–1327.
Acknowledgements
We thank McArdle Laboratories for Cancer Research for support of this project. We also thank the UW Carbone Comprehensive Cancer Center (UWCCC) for use of its shared services to complete this research. We thank Drs Pierre Chambon, Vladimir Spiegelman, Robert Kalejta and Greg Finn for providing us appropriate expression plasmids and reagents. We also thank Dr Wei Xu for insightful comments. This work was supported by NIH grants CA159578 (to E.T.A.) and T32 CA009135 (to P.R.) and a sponsored research award from Genoptix/Novartis (to D.R.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Rajbhandari, P., Schalper, K., Solodin, N. et al. Pin1 modulates ERα levels in breast cancer through inhibition of phosphorylation-dependent ubiquitination and degradation. Oncogene 33, 1438–1447 (2014). https://doi.org/10.1038/onc.2013.78
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2013.78
Keywords
This article is cited by
-
Overexpression of TPL2 may be a predictor of good prognosis in patients with breast invasive ductal carcinoma
Scientific Reports (2023)
-
SMURF1 facilitates estrogen receptor ɑ signaling in breast cancer cells
Journal of Experimental & Clinical Cancer Research (2018)
-
Prolyl isomerase Pin1: a promoter of cancer and a target for therapy
Cell Death & Disease (2018)
-
Association of B7-H4, PD-L1, and tumor infiltrating lymphocytes with outcomes in breast cancer
npj Breast Cancer (2018)
-
CCN5/WISP-2 restores ER-∝ in normal and neoplastic breast cells and sensitizes triple negative breast cancer cells to tamoxifen
Oncogenesis (2017)