Abstract
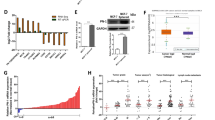
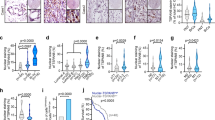
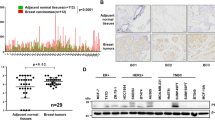
Metastasis is responsible for more than 90% of the mortality observed among patients with breast cancer. Human phosphatidylethanolamine-binding protein 4 (hPEBP4) is a novel member of the PEBP family and functions as an anti-apoptotic molecule. Here, we found that the metastatic MDA-MB-231 breast cancer cells expressed much higher levels of hPEBP4 than the nonmetastatic MCF-7 breast cancer cells and that the expression levels of hPEBP4 were positively correlated with the metastasis of clinical breast cancer. The hPEBP4 overexpression in the MDA-MB-231 cells significantly promoted cell invasion in vitro and increased the development of lymph node metastasis in vivo. Conversely, the silencing of hPEBP4 suppressed the cell-invasive ability both in vitro and in vivo. Further investigation showed that hPEBP4 promoted the expression or activity of the metastasis-related proteinases MMP (matrix metalloproteinase) 2, MMP9 and MMP13. This hPEBP4-potentiated cell invasion and MMP expression is due to an increase in Akt activation. Knockdown of Akt restored hPEBP4-induced breast tumor metastasis in the hPEBP4-MDA-MB-231 xenograft mouse model. Moreover, we found that hPEBP4 functioned as a scaffolding molecule and enhanced the association of Akt with Src to promote Akt tyrosine phosphorylation, a prerequisite for the full activation of Akt, in a phosphatidylethanolamine-binding domain-dependent manner. Given the present information about human breast cancer, these functional data from cell culture and animal studies suggest that, in human breast cancer hPEBP4 is a novel and clinically relevant metastasis accelerator gene and may be a new diagnostic marker and therapeutic target for breast cancer metastasis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Weigelt B, Peterse JL, van’t Veer LJ . Breast cancer metastasis: markers and models. Nat Rev Cancer 2005; 5: 591–602.
Qiao M, Sheng S, Pardee AB . Metastasis and AKT activation. Cell Cycle 2008; 7: 2991–2996.
Prasad S, Ravindran J, Aggarwal BB . NF-kappaB and cancer: how intimate is this relationship. Mol Cell Biochem 2010; 336: 25–37.
Min C, Eddy SF, Sherr DH, Sonenshein GE . NF-κB and epithelial to mesenchymal transition of cancer. J Cell Biochem 2008; 104: 733–744.
Al Saleh S, Sharaf LH, Luqmani YA . Signalling pathways involved in endocrine resistance in breast cancer and associations with epithelial to mesenchymal transition. Int J Onco 2011; 38: 1197–1217.
Wang X, Li N, Liu B, Sun H, Chen T, Li H et al. A novel human phosphatidylethanolamine-binding protein resists tumor necrosis factor α-induced apoptosis by inhibiting mitogen-activated protein kinase pathway activation and phosphatidylethanolamine externalization. J Biol Chem 2004; 279: 45855–45864.
Wang X, Li N, Li H, Liu B, Qiu J, Chen T et al. Silencing of human phosphatidylethanolamine-binding protein 4 sensitizes breast cancer cells to tumor necrosis factor-α–induced apoptosis and cell growth arrest. Clin Cancer Res 2005; 11: 7545–7553.
Li H, Wang X, Li N, Qiu J, Zhang Y, Cao X . hPEBP4 resists TRAIL-induced apoptosis of human prostate cancer cells by activating Akt and deactivating ERK1/2 pathways. J Biol Chem 2007; 282: 4943–4950.
Li P, Li N, Kong H, Guo Z, Liu S, Cao X . Anti-apoptotic hPEBP4 silencing promotes TRAIL-induced apoptosis of human ovarian cancer cells by activating ERK and JNK pathways. Int J Mol Med 2006; 18: 505–510.
Garcia R, Grindlay J, Rath O, Fee F, Kolch W . Regulation of human myoblast differentiation by PEBP4. EMBO Rep 2009; 10: 278–284.
Liu H, Kong Q, Li B, He Y, Li P, Jia B . Expression of PEBP4 protein correlates with the invasion and metastasis of colorectal cancer. Tumor Biol 2012; 33: 267–273.
Yu G-P, Chen G-Q, Wu S, Shen K, Ji Y . The expression of PEBP4 protein in lung squamous cell carcinoma. Tumor Biol 2011; 32: 1257–1263.
Yu G-P, Huang B, Chen G-Q, Wu S, Ji Y, Shen Z-Y . PEBP4 gene expression and its significance in invasion and metastasis of non-small cell lung cancer. Tumor Biol 2012; 33: 223–228.
Yu G, Shen Z, Chen G, Teng X, Hu Y, Huang B . PEBP4 enhanced HCC827 cell proliferation and invasion ability and inhibited apoptosis. Tumor Biol 2012; 34: 91–98.
Zhang Y, Wang X, Xiang Z, Li H, Qiu J, Sun Q et al. Promotion of cellular migration and apoptosis resistance by a mouse eye-specific phosphatidylethanolamine-binding protein. Int J Mol Med 2007; 19: 55–63.
Kohn AD, Takeuchi F, Roth RA . Akt a pleckstrin homology domain containing kinase, is activated primarily by phosphorylation. J Biol Chem 1996; 271: 21920–21926.
Yang W-L, Wu C-Y, Wu J, Lin H-K . Regulation of Akt signaling activation by ubiquitination. Cell Cycle 2010; 9: 486–497.
Luan B, Zhao J, Wu H, Duan B, Shu G, Wang X et al. Deficiency of a [bgr]-arrestin-2 signal complex contributes to insulin resistance. Nature 2009; 457: 1146–1149.
Jiang T, Qiu Y . Interaction between Src and a C-terminal proline-rich motif of Akt is required for Akt activation. J Biol Chem 2003; 278: 15789–15793.
Chen R, Kim O, Yang J, Sato K, Eisenmann KM, McCarthy J et al. Regulation of Akt/PKB activation by tyrosine phosphorylation. J Biol Chem 2001; 276: 31858–31862.
Zheng Y, Peng M, Wang Z, Asara JM, Tyner AL . Protein tyrosine kinase 6 directly phosphorylates AKT and promotes AKT activation in response to epidermal growth factor. Mol Cell Biol 2007; 30: 4280–4292.
Zhu QS, Rosenblatt K, Huang KL, Lahat G, Brobey R, Bolshakov S et al. Vimentin is a novel AKT1 target mediating motility and invasion. Oncogene 2011; 30: 457–470.
Roskoski R Jr . Src protein–tyrosine kinase structure and regulation. Biochem Biophys Res Commun 2004; 324: 1155–1164.
Yeatman TJ . A renaissance for SRC. Nat Rev Cancer 2004; 4: 470–480.
Castaneda CA, Cortes-Funes H, Gomez HL, Ciruelos EM . The phosphatidyl inositol 3-kinase/AKT signaling pathway in breast cancer. Cancers Metastasis Rev 2010; 29: 751–759.
López-Knowles E, O’Toole SA, McNeil CM, Millar EK, Qiu MR, Crea P et al. PI3K pathway activation in breast cancer is associated with the basal-like phenotype and cancer-specific mortality. Int J Cancer 2010; 126: 1121–1131.
Guertin DA, Sabatini DM . Defining the role of mTOR in cancer. Cancer Cell 2007; 12: 9–22.
Cantley LC . The phosphoinositide 3-kinase pathway. Science 2002; 296: 1655–1657.
Yang W-L, Wang J, Chan C-H, Lee S-W, Campos AD, Lamothe B et al. The E3 ligase TRAF6 regulates Akt ubiquitination and activation. Science 2009; 325: 1134–1138.
Liu H, Qiu J, Li N, Chen T, Cao X . Human phosphatidylethanolamine-binding protein 4 promotes transactivation of estrogen receptor α (ERα) in human cancer cells by inhibiting proteasome-dependent ERα degradation via association with Src. J Biol Chem 2010; 285: 21934–21942.
Odabaei G, Chatterjee D, Jazirehi AR, Goodglick L, Yeung K, Bonavida B . Raf-1 kinase inhibitor protein: structure, function, regulation of cell signaling, and pivotal role in apoptosis. Adv Cancer Res 2004; 91: 169–200.
Baritaki S, Katsman A, Chatterjee D, Yeung KC, Spandidos DA, Bonavida B . Regulation of tumor cell sensitivity to TRAIL-induced apoptosis by the metastatic suppressor Raf kinase inhibitor protein via yin yang 1 inhibition and death receptor 5 up-regulation. J Immunol 2007; 179: 5441–5453.
Chatterjee D, Bai Y, Wang Z, Beach S, Mott S, Roy R et al. RKIP sensitizes prostate and breast cancer cells to drug-induced apoptosis. J Biol Chem 2004; 279: 17515–17523.
Fu Z, Smith PC, Zhang L, Rubin MA, Dunn RL, Yao Z et al. Effects of Raf kinase inhibitor protein expression on suppression of prostate cancer metastasis. J Natl Cancer Inst 2003; 95: 878–889.
Dangi-Garimella S, Yun J, Eves EM, Newman M, Erkeland SJ, Hammond SM et al. Raf kinase inhibitory protein suppresses a metastasis signalling cascade involving LIN28 and let-7. EMBO J 2009; 28: 347–358.
Hagan S, Al-Mulla F, Mallon E, Oien K, Ferrier R, Gusterson B et al. Reduction of Raf-1 kinase inhibitor protein expression correlates with breast cancer metastasis. Clin Cancer Res 2005; 11: 7392–7397.
Ma L, Teruya-Feldstein J, Weinberg RA . Tumour invasion and metastasis initiated by microRNA-10b in breast cancer. Nature 2007; 449: 682–688.
Cheung LWT, Leung PCK, Wong AS . Gonadotropin-releasing hormone promotes ovarian cancer cell invasiveness through c-Jun NH2-terminal kinase–mediated activation of matrix metalloproteinase (MMP)-2 and MMP-9. Cancer Res 2006; 66: 10902–10910.
Wang Y, Zhang H, Chen Y, Sun Y, Yang F, Yu W et al. LSD1 is a subunit of the NuRD complex and targets the metastasis programs in breast cancer. Cell 2009; 138: 660–672.
Acknowledgements
This work was supported by grants from the National Basic Research Program of China (973) (2014CB542101, 2013CB530502), the National Natural Science Foundation of China (81282353, 81072437, 81000302), the Doctoral Fund of Ministry of Education of China (20120101110022), Zhejiang Major Science and Technology Special Project (2009C13018, 2009C14015), the Research Fund of Science Base (J1103603), Qianjiang Talent Program of Zhejiang Province (2012R10027) and Scientific Research Foundation of the Health Bureau of Zhejiang Province (WKJ2012-2-030).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Li, H., Huang, F., Fan, L. et al. Phosphatidylethanolamine-binding protein 4 is associated with breast cancer metastasis through Src-mediated Akt tyrosine phosphorylation. Oncogene 33, 4589–4598 (2014). https://doi.org/10.1038/onc.2013.408
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2013.408
Keywords
This article is cited by
-
SRC kinase-mediated signaling pathways and targeted therapies in breast cancer
Breast Cancer Research (2022)
-
MPPa-PDT suppresses breast tumor migration/invasion by inhibiting Akt-NF-κB-dependent MMP-9 expression via ROS
BMC Cancer (2019)
-
NEMP1 Promotes Tamoxifen Resistance in Breast Cancer Cells
Biochemical Genetics (2019)
-
ETV4 transcription factor and MMP13 metalloprotease are interplaying actors of breast tumorigenesis
Breast Cancer Research (2018)
-
Facial cutaneo-mucosal venous malformations can develop independently of mutation of TEK gene but may be associated with excessive expression of Src and p-Src
Journal of Negative Results in BioMedicine (2017)