Abstract
Increased microRNA-10b (miR-10b) expression in the cancer cells in pancreatic ductal adenocarcinoma (PDAC) is a marker of disease aggressiveness. In the present study, we determined that plasma miR-10b levels are significantly increased in PDAC patients by comparison with normal controls. By gene profiling, we identified potential targets downregulated by miR-10b, including Tat-interacting protein 30 (TIP30). Immunoblotting and luciferase reporter assays confirmed that TIP30 was a direct miR-10b target. Downregulation of TIP30 by miR-10b or siRNA-mediated silencing of TIP30 enhanced epidermal growth factor (EGF)-dependent invasion. The actions of miR-10b were abrogated by expressing a modified TIP30 cDNA resistant to miR-10b. EGF-induced EGF receptor (EGFR) tyrosine phosphorylation and extracellular signal–regulated kinase phosphorylation were enhanced by miR-10b, and these effects were mimicked by TIP30 silencing. The actions of EGF in the presence of miR-10b were blocked by EGFR kinase inhibition with erlotinib and by dual inhibition of PI3K (phosphatidylinositol 3′-kinase) and MEK. Moreover, miR-10b, EGF and transforming growth factor-beta (TGF-β) combined to markedly increase cell invasion, and this effect was blocked by the combination of erlotinib and SB505124, a type I TGF-β receptor inhibitor. miR-10b also enhanced the stimulatory effects of EGF and TGF-β on cell migration and epithelial–mesenchymal transition (EMT) and decreased the expression of RAP2A, EPHB2, KLF4 and NF1. Moreover, miR-10b overexpression accelerated pancreatic cancer cell (PCC) proliferation and tumor growth in an orthotopic model. Thus, plasma miR-10b levels may serve as a diagnostic marker in PDAC, whereas intra-tumoral miR-10b promotes PCC proliferation and invasion by suppressing TIP30, which enhances EGFR signaling, facilitates EGF–TGF-β cross-talk and enhances the expression of EMT-promoting genes, whereas decreasing the expression of several metastasis-suppressing genes. Therefore, therapeutic targeting of miR-10b in PDAC may interrupt growth-promoting deleterious EGF–TGF-β interactions and antagonize the metastatic process at various levels.
Similar content being viewed by others
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is the fourth leading cause of cancer-related deaths in the United States with a median survival of 6 months and a 5-year survival rate of 6%.1 As there are no effective modalities for early detection, most PDAC patients present with locally invasive and/or metastatic disease and are not candidates for resection.2 Histologically, PDAC is characterized by regions of acinar-to-ductal metaplasia, pancreatic intra-epithelial neoplasia, cancer cells forming ductal-like structures, inflammatory infiltrates and an extensive desmoplastic reaction that interferes with effective drug delivery into the pancreatic tumor mass.3, 4, 5 At the molecular level, there is a high frequency of major driver mutations in key regulatory genes, including KRAS, TP53, CDKN2A and SMAD4, an abundance of low-frequency driver mutations and aberrant activation of multiple signaling pathways as a consequence of the overexpression of multiple tyrosine kinase receptors, such as the epidermal growth factor (EGF) receptor (EGFR) and its ligands, and excessive production of transforming growth factor-beta (TGF-β) isoforms.6, 7
EGFR homodimerization and heterodimerization with other EGFR family members leads to enhanced tyrosine kinase activity of the receptor complexes and activation of multiple signaling pathways, such as mitogen-activated protein kinase (MAPK), p38 MAPK, phosphatidylinositol 3′-kinase (PI3K), Src, Crk and Nck.8, 9 By contrast, TGF-β activates serine-threonine receptor kinases, which act through canonical, Smad-dependent signaling as well as non-canonical, Smad-independent signaling, and generally exert paracrine effects on the tumor microenvironment in PDAC.10, 11, 12 However, TGF-β can also directly enhance the invasion of pancreatic cancer cells (PCCs) and stimulate PCC proliferation,13 indicating that EGF–TGF-β interactions are important with respect to cell-autonomous effects in PDAC.
MicroRNAs (miRs) constitute a class of 18–25 nucleotide non-coding RNAs that target specific mRNA moieties for translational repression or degradation.14 Expression of miRs is often deregulated in cancer, and miRs participate in the regulation of many important biological processes, including cell survival, proliferation, migration, invasion and metastasis.15, 16 Several miRs are overexpressed in PDAC, including miR-10b, miR-21, miR-196a, miR-203, miR-155, miR-210 and miR-221.17, 18, 19, 20, 21, 22 Moreover, miR-10b is one of the most frequently upregulated miRs in PDAC,18, 22 and high miR-10b levels in the cancer cells in PDAC are associated with decreased therapeutic response to multimodality neoadjuvant therapy, shorter time to metastasis, decreased overall patient survival and increased PCC invasiveness.22, 23, 24 However, it is not clear how miR-10b promotes PCC invasion in PDAC.
In the present study, we determined that miR-10b enhanced EGF-induced invasion, EGFR phosphorylation and extracellular signal–regulated kinase 1 (ERK1)/2 activation in PCCs, whereas attenuating EGFR degradation and that these effects were due to miR-10b-induced downregulation of Tat-interacting protein 30 (TIP30). Moreover, the combination of high levels of miR-10b, EGF and TGF-β markedly enhanced cell invasion and altered gene expression in a manner that was consistent with epithelial–mesenchymal transition (EMT) induction. Given that miR-10b, EGFR and TGF-β are often overexpressed in PDAC,22, 23, 24, 25, 26 these observations suggest that targeting miR-10b may serve to suppress metastases and to interrupt deleterious EGF–TGF-β interactions in PDAC.
Results
miR-10b is expressed at high levels in the cancer cells in PDAC,18, 22 and several studies have implicated miR-10b in cancer metastasis.27, 28 To confirm that miR-10b expression is increased in PDAC, we took advantage of the known resistance of miRs to degradation in the circulation and assayed miR-10b levels in the plasma of 20 normal controls, 5 patients with chronic pancreatitis and 17 PDAC patients. miR-10b levels were increased 575-fold in PDAC patients by comparison with the corresponding levels in either normal controls (P<0.001) or patients with chronic pancreatitis (Figure 1a; P<0.001).
miR-10b targets. (a) Plasma miR-10b levels. Compared with plasma from normal controls (n=20; closed circles) and chronic pancreatitis patients (CP; n=5; gray circles), miR-10b levels are significantly elevated (P<.001) in the plasma of PDAC patients (n=18; open circles). Horizontal bars denote mean expression levels. (b) Gene expression. qRT–PCR of indicated mRNAs in PANC-1 cells transfected with control (solid bars) or precursor miR-10b (open bars) confirmed the array results. (c) TIP30 levels. qRT–PCR was used to determine TIP30 mRNA levels in PANC-1 cells transfected with control (solid bars) or precursor miR-10b (open bars) for the indicated times. (d) TIP30 immunoblotting. A highly specific anti-TIP30 antibody was used in immunoblotting analysis, revealing a marked decrease in TIP30 protein levels in the presence of miR-10b by comparison with control. (e) Luciferase reporter constructs. The reporter constructs encoded a wild-type (WT) TIP30 3′UTR or a mutant (M) TIP30 3′UTR in which the binding site was replaced by the indicated nucleotides. (f) Luciferase readout. PANC-1 cells were transfected with control (solid bars) or precursor miR-10b (open bars) for 20 h together with WT or M TIP30 3′UTR luciferase constructs. Data in panels b, c, and f, are the means±s.e.m. from three experiments, analyzed by two-way ANOVA followed by Bonferroni’s adjustment. *P<0.01 and **P<0.001 compared with its respective controls.
Given that the metastatic process is preceded by invasion, we sought to identify invasion-associated miR-10b targets that could lead to enhanced metastasis in PDAC. Accordingly, microarray studies were conducted using RNA from PANC-1 cells transfected with a miR-10b precursor. Fifty-five genes were downregulated by at least 40% upon miR-10b overexpression (Supplementary Table S1), and three of these genes (RAP2A, EPHB2 and TIP30) affect pathways that have the potential to suppress cancer cell invasion. Quantitative reverse transcription PCR (qRT–PCR) confirmed that the mRNA levels of all three genes were significantly decreased in PANC-1 cells expressing high levels of miR-10b compared with respective controls (Figure 1b). In silico analysis with miRanda, PicTar and TargetScan computational tools for miRNA target prediction confirmed that TIP30, RAP2A and EPHB2 were potential miR-10b targets.
TIP30, also known as HIV-1 Tat interactive protein 2 (HTATIP2) or CC3, was of particular interest, because it suppresses metastases in lung, breast and hepatocellular cancers29, 30, 31 and regulates EGF-induced EGFR endocytosis.32 Given the important role of EGFR in PDAC,25, 33 we sought to confirm that miR-10b downregulates TIP30 mRNA and protein levels. Accordingly, qRT–PCR and immunoblotting were conducted using RNA and protein extracted from miR-10b-overexpressing PANC-1 cells. Increased miR-10b expression led to decreased TIP30 mRNA (Figure 1c) and protein (Figure 1d) levels at 24, 48 and 72 h post transfection. A similar decrease in TIP30 protein levels was seen in ASPC-1, COLO-357 and T3M4 human PCCs following transfection with miR-10b precursor (Supplementary Figure S1a), indicating that this downregulation was not unique to PANC-1 cells. Moreover, transfection with the miR-10a precursor, which shares the same sequence with miR-10b except for a difference of one nucleotide in the non-seed region,34 resulted in variable but reproducible decreases in TIP30 levels in all the four PCCs (Supplementary Figure S1a).
To confirm that TIP30 is a direct target of miR-10b, we used a TIP30 3′ untranslated region (UTR) luciferase construct, and a mutant construct in which 6 nucleotides in the potential binding site were replaced (Figure 1e). Co-transfection of PANC-1 cells with the TIP30 3′UTR construct and miR-10b precursor caused a 50% decrease in luciferase activity compared with the negative control, and this suppression of TIP30 expression was completely reversed by the six-nucleotide substitution in the core binding site (Figure 1f).
EGF and TGF-β promote PCC invasion.12 To determine whether miR-10b enhances human PCC invasion and whether it modulates the actions of either EGF or TGF-β, miR-10b precursor was transfected into two human PCCs. In PANC-1 and COLO-357 cells, EGF (1 nM) or TGF-β1 (0.5 nM) enhanced invasion, and their combined action was greater than that of either growth factor alone (Figures 2a and b). Overexpression of miR-10b increased invasion in PANC-1 cells and enhanced EGF-mediated invasion in both PCCs and TGF-β1-mediated invasion in COLO-357 cells. Moreover, the combination of both growth factors exerted a significantly (P<0.005) greater stimulatory effect on invasion in cells with high miR-10b levels (Figures 2a and b), which is potentially important clinically given that miR-10b, EGFR and TGF-β are all overexpressed in PDAC.22, 23, 24, 25, 26
Effects of miR-10b, EGF and TGF-β1 on invasion and migration, and consequences of TIP30 downregulation on invasion. (a, b) Effects of miR-10b, EGF and TGF-β1 on invasion. COLO-357 (a) and PANC-1 (b) cells were transfected with control (solid bars) or miR-10b (open bars) precursors and plated (5 × 104 cells/well) in a Matrigel-coated Boyden chamber. Invasion was determined after incubating cells for 20 h in the absence (serum free: SF) or presence of EGF (E, 1 nM), TGF-β1 (T, 0.5 nM) or both EGF and TGF-β1 (E+T). Cells that had invaded across the membrane were stained and counted. (c) TIP30 silencing. PANC-1 cells were transfected with scrambled control or two siRNAs (5 and 6) targeting TIP30. Cells (5 × 104 cells per well) were then plated for Matrigel invasion assays in the absence or presence of EGF (1 nM) for 20 h. (d) Effects of mutant TIP30 on miR-10b-enhanced invasion. PANC-1 cells were transfected for 48 h with control or precursor miR-10b in combination with either empty pCMV-SPORT6 (Empty) or pCMV-SPORT6-TIP30 carrying a mutated TIP30 cDNA(TIP30 Mutant) that is resistant to miR-10b-induced downregulation. Effects on invasion by 1 nM EGF were then determined. Data in each panel are the means±s.e.m. from three experiments and were analyzed by two-way ANOVA followed by Bonferroni’s adjustment. *P<0.05, **P<0.01 and ***P<0.001 compared with respective controls; #P<0.05, compared with corresponding control SF; †P<0.01 compared with EGF alone and ‡P<0.01 compared with TGF-β1 alone in cells transfected with miR-10b precursor; †P<0.05 compared with empty pCMV-SPORT6, and *P<0.05 compared with pCMV-SPORT6-TIP30 in miR-10b-transfected group. (e, f) Migration assays. COLO-357 (e) and PANC-1 (f) cells were transfected with control (solid bars) or miR-10b precursors (open bars). Cells (5 × 104 cells/well) were then used in a wound-healing assay and incubated for 20 h in the absence (SF) or presence of EGF (E, 1 nM), TGF-β1 (T, 0.5 nM) or both EGF and TGF-β1 (E+T). The wound area was measured at 0 and 20 h using ImageJ software (NIH, Bethedsa, MD, USA). Migration was calculated as the percentage of change in wound area. Two-way ANOVA was performed followed by Bonferroni’s adjustment. **P<0.01 and ***P<0.001 compared with respective controls; #P<0.001 compared with control SF; †P<0.05 and †††P<0.001 compared with EGF alone in cells transduced with miR-10b; ‡P<0.05 and ‡‡‡P<0.001 compared with TGF-β1 alone in cells transduced with miR-10b.
To determine whether TIP30 knockdown mimics miR-10b actions on PCC invasion, PANC-1 cells were transfected with two siRNAs targeting TIP30. TIP30 silencing by either siRNA reduced TIP30 levels (Supplementary Figure S1b) and increased EGF-stimulated invasion (Figure 2c), indicating that TIP30 is a negative regulator of PCC invasion. To confirm that TIP30 downregulation was essential for miR-10b-induced stimulation of cell invasion, we used the pCMV-SPORT6-TIP30 vector, which encodes a TIP30 cDNA that is not regulated by miR-10b due to a mutated 3′UTR binding site. Experiments were carried out in the presence of 1 nM EGF and in the absence or presence of transfected miR-10b using cells expressing empty vector or the pCMV-SPORT6-TIP30 vector. This experimental design allowed for the specific evaluation of the consequence of expressing a TIP30 that was resistant to miR-10b downregulation. When pCMV-SPORT6-TIP30 was transfected into PANC-1 cells, the stimulatory effect of miR-10b overexpression on EGF-induced invasion was completely abrogated (Figure 2d). Thus, TIP30 is a functional target of miR-10b whose downregulation promotes EGF-induced invasion.
Invasion is often associated with enhanced cancer cell motility. Therefore, we next sought to determine whether miR-10b modulated PCC migration. In COLO-357 cells, miR-10b, EGF (1 nM), TGF-β1 (0.5 nM) or the combination of EGF and TGF-β1 also increased cell migration (Figure 2e). Moreover, in the presence of miR-10b, the combined effect of EGF and TGF-β1 on migration was significantly greater than with either growth factor alone (Figure 2e). By contrast, in PANC-1 cells EGF enhanced migration only following miR-10b transfection, whereas TGF-β1 and the combination of EGF and TGF-β1 significantly increased migration in control cells, and these effects were enhanced in the presence of miR-10b (Figure 2f).
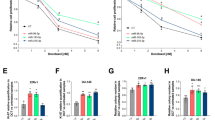
Specific inhibitors were used next to confirm that EGF and TGF-β acted through their respective receptors with respect to their individual and combined stimulatory effects on invasion in the presence of miR-10b. The EGFR inhibitor erlotinib (2 μM) completely blocked EGF-induced invasion in the presence of miR-10b in both the COLO-357 and PANC-1 cells (Figures 3a and b). Although the PI3K inhibitor LY294002 (10 μM) and the MEK inhibitor UO126 (1 μM) partially blocked this effect, the combination of LY294002 and UO126 were as effective as erlotinib in suppressing EGF-mediated invasion (Figures 3a and b). Moreover, miR-10b’s effects on combined EGF–TGF-β1 actions on invasion were markedly attenuated by EGFR kinase inhibition with erlotinib and TβRI kinase inhibition with SB505124 and were abrogated by their combined actions (Figures 3c and d), indicating that EGF and TGF-β1 act through their respective receptors to cross-talk and enhance invasion.
Effects of signaling inhibitors on invasion and effects of miR-10b on signaling. (a, b) Effects of EGFR inhibitor erlotinib and downstream inhibitors on invasion. COLO-357 (a) and PANC-1 (b) cells were transfected with miR-10b precursor were incubated for 20 h without EGF (SF) or with EGF (1 nM) in the absence (D: DMSO) or presence of 2 μM erlotinib (E), 10 μM LY294002 (LY: PI3K inhibitor) or 1 μM UO126 (U: MEK inhibitor). (c, d) Effects of erlotinib and TβRI inhibition with SB505124 on invasion. COLO-357 (c) and PANC-1 (d) cells transfected with miR-10b precursor were incubated for 20 h without growth factor (−) or with 1 nM EGF and 0.5 nM TGF-β1 (+) in the absence (D: DMSO) or presence of erlotinib (E, 2 μM) or SB505124 (SB, 10 μM), and effects on invasion were determined. Data in all panels are the means±s.e.m. from three experiments and were analyzed by ANOVA followed by Bonferroni’s adjustment. In panels (a) and (b), *P<0.05, **P<0.01 and ***P<0.001 compared with EGF alone; in panels (c) and (d) ***P<0.001 compared with EGF and TGF-β1 combination and †P<0.05 compared with corresponding serum free (SF) values. (e, f) Effects of miR-10b overexpression and TIP30 knockdown on EGFR signaling. (e) PANC-1 cells transfected with control or miR-10b precursor were incubated in the absence of presence of 1 nM EGF for the indicated times. Immunoblotting for the indicated proteins was then carried out. ERK2 was used to assess loading of lanes. (f) Effects of TIP30 silencing on EGFR signaling. PANC-1 cells transfected with control siRNA or siRNA targeting TIP30 were incubated in the absence or presence of 1 nM EGF for the indicated times. Immunoblotting for the indicated proteins was then carried out, ERK2 serving to assess loading of lanes.
TIP30 is a crucial component of the complex that regulates endocytotic trafficking of EGFR in breast and liver cancer cells and may act to prolong downstream signaling.31, 32 We therefore sought to determine whether miR-10b modulates EGFR expression and signaling in PANC-1 cells. In control-transfected cells, EGF caused a gradual decrease in EGFR protein levels, and this effect was attenuated in miR-10b-overexpressing cells (Figure 3e) but was not associated with any alteration in EGFR mRNA levels (not shown). EGF also rapidly increased EGFR (Tyr 1148) and ERK1/2 phosphorylation (Thr-202/Tyr-204), and both of these effects were enhanced by miR-10b (Figure 3e). By contrast, EGF-induced AKT phosphorylation (Ser 473) and p38 MAPK phosphorylation (Thr-180/Tyr-182) were not altered by miR-10b (Figure 3e).
To assess whether downregulation of TIP30 alone is sufficient to modulate EGFR signaling in PCCs, TIP30 levels were suppressed with siRNA 6 (Supplementary Figure S1b). TIP30 knockdown attenuated EGF-induced EGFR downregulation and enhanced EGFR and ERK1/2 phosphorylation without altering p38 MAPK phosphorylation (Figure 3f). In contrast to the actions of miR-10b, siRNA targeting of TIP30 enhanced EGF-induced AKT phosphorylation (Figure 3f), suggesting that TIP30 may suppress pro-survival signals independently of its actions on EGFR downregulation.
Cancer cell invasion is often associated with enhanced EMT, and TGF-β1 induces EMT in PCCs.35 Accordingly, we next sought to determine whether miR-10b modulated the effects of TGF-β and EGF on the expression of EMT-associated genes (Figure 4). In both the COLO-357 and PANC-1 cells, miR-10b significantly decreased E-cadherin levels, and this effect was most pronounced in the presence of the combination of TGF-β1 and EGF (Figure 4a). In both the cell lines, EGF plus TGF-β1 increased N-cadherin and vimentin expression but only in the presence of high miR-10b levels (Figures 4b and c). Moreover, in the presence of miR-10b, N-cadherin expression was increased by TGF-β1 alone in COLO-357 cells and by EGF alone in PANC-1 cells, where EGF plus TGF-β1 also increased N-cadherin in the absence of added miR-10b (Figure 4b). Snail and Zeb1 expression was increased in COLO-357 cells in response to either or both the growth factors in the presence of miR-10b, as well as in the presence of both the growth factors without added miR-10b (Figures 4d and e). By contrast, PANC-1 cells exhibited increased Snail levels only in response to both the growth factors, and this effect was increased slightly by miR-10b (Figure 4d). Increases in Zeb1 expression in PANC-1 cells paralleled those observed in COLO-357 cells, except that TGF-β1 was effective even in the absence of added miR-10b (Figure 4e).
Effects of miR-10b on expression of genes implicated in EMT. (a, e) qRT–PCR. Control (solid bars) or miR-10b precursor (open bars) transfected COLO-357 and PANC-1 cells were incubated in the absence (serum free: SF) or presence of EGF (E, 1 nM), TGF-β1 (T, 0.5 nM) or both EGF and TGF-β1 (E+T) for 24 h and then harvested for RNA extraction and qRT–PCR for E-cadherin (a), N-cadherin (b), Vimentin (c), Snail (d) and Zeb1 (e). Data are the means±s.e.m. from four experiments. Two-way ANOVA was performed followed by Bonferroni’s adjustment. *P<0.05, **P<0.01 and ***P<0.001 compared with respective control transfected cells; #P<0.05, ##P<0.01, and ###P<0.001 compared with respective cells in SF condition.
We next examined the effects of miR-10b on the expression of several known miR-10b target genes,27, 36, 37, 38, 39, 40 HOXD10, TIAM1, KLF4, SDC1 and NF1 in AsPC-1, COLO-357 and PANC-1 cells. By qRT–PCR, KLF4, SDC1 and NF1 expression was decreased by miR-10b in all the three cell lines (Figure 5a), whereas NF2 expression was not altered (Figure 5a). Moreover, miR-10b did not alter the expression of either HOXD10 or TIAM1 (not shown).
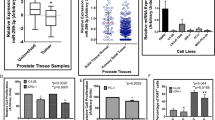
miR-10b targets in human PCCs and TIP30 expression in PDAC. (a) qRT–PCR. AsPC-1, COLO-357 and PANC-1 cells were transfected with control (solid bars) or miR-10b precursor (open bars) for 48 h and then harvested for RNA extraction and qRT–PCR for the indicated mRNAs. (b, c) TIP30 expression in PDAC. Immunohistochemistry for TIP30 in human PDAC tissues shows that TIP30 exhibits strong immunoreactivity in the PCCs in well to moderately differentiated cancers (b). By contrast, the PCCs in poorly differentiated PDACs exhibit weak TIP30 immunoreactivity (c). Insets show magnified images of boxed areas. Representative images are shown. Bars=50 μm. *P<0.05, compared with respective control transfected cells.
We next sought to determine whether TIP30 was expressed in human PDAC samples using the same highly specific antibody that was used in the immunoblotting experiments. TIP30 was readily visible in the cytoplasm of many cancer cells but not in the adjoining stroma. Moreover, high TIP30 expression was principally seen in well to moderately differentiated PDAC (Figure 5b), whereas low TIP30 expression was seen in poorly differentiated PDAC (Figure 5c).
To validate the contribution of miR-10b to PDAC biological aggressiveness, we carried out an in vivo study in an orthotopic model using athymic mice (Figure 6) and T3M4 cells engineered to stably overexpress miR-10b. T3M4 cells were used for these studies as they express high EGFR levels41 and low miR-10b levels (not shown). Both control and miR-10b-overexpressing T3M4 cells formed intra-pancreatic tumors in all mice, but the presence of high levels of miR-10b resulted in faster growing and larger tumors (Figures 6a and b). Although the two tumor groups were similar histologically, miR-10b-overexpressing tumors exhibited many Ki67-positive cells reflecting the presence of a large number of cells in late G1, S, G2 or M phase (Figure 6c). Moreover, there was a marked increase in the number of phospho-histone H3-positive cancer cells that are undergoing mitosis in the miR-10b-overexpressing tumors by comparison with control tumors (Figure 6c). Thus, miR-10b exerts multiple deleterious actions in PCCs, which include upregulation of EGFR signaling, downregulation of NF-1 and decreased expression of metastasis-suppressing genes, thereby contributing to increased PCC proliferation and invasion (Figure 6d).
miR-10b overexpression enhances tumor growth. (a, b) Effects of miR-10b overexpression on T3M4 growth in vivo. (a) High-resolution ultrasound images obtained 3 weeks post-PCC injection into the pancreas show that compared with cells transduced with an empty vector (control), miR-10b transduction dramatically enhances tumor growth. Tumors are outlined and shown are representative images from two of five control or miR-10b-overexpressing tumors. (b) Quantitation of these tumors, calculated via three-dimensional abdominal imaging, shows that miR-10b overexpression (open bar) significantly enhances tumor volume. Data are the means±s.e.m. for all five control (Sham) and miR-10b-transduced tumors. A t-test was used to test for significant differences. *P<0.05. (c) Effects of miR-10b overexpression on proliferation in vivo. Immunohistochemistry of control and miR-10b-transduced tumors shows that miR-10b overexpression enhances proliferation as evidenced by the increased Ki67 and phospho-Histone H3 immunoreactivity when compared with control tumors. Shown are representative H&E stains, and Ki67 and phospho-Histone H3 immunostaining from one of five control or miR-10b-transduced tumors. Bars=50 μm. (d) Schematic representation of miR-10b actions in PCCs.
Discussion
miR-10b was initially reported as a metastasis promoter in breast cancer27 and subsequently implicated in enhancing metastasis in cancers of the esophagus,37 colon,42 liver43 and pancreas24 and invasion in glioblastoma.38 In breast cancer, miR-10b downregulates HOXD10 leading to the increased expression of the metastasis-promoting RhoC.27 Moreover, in a syngeneic orthotopic mouse model of breast cancer, miR-10b antagomirs significantly attenuated the frequency of metastases to the lungs.28
In the present study, we determined that plasma miR-10b levels are markedly increased in PDAC patients by comparison with either normal subjects or patients with chronic pancreatitis, suggesting that assaying for miR-10b could potentially be a useful diagnostic biomarker. Moreover, miR-10b overexpression in PCCs enhanced EGF-stimulated invasion. By gene profiling, immunoblotting and luciferase reporter assays, TIP30 was identified as a target directly downregulated by miR-10b overexpression. Four lines of evidence suggested that miR-10b promoted EGF-mediated invasion by downregulating TIP30 and enhancing EGFR signaling. First, the expression of a TIP30 cDNA resistant to silencing by miR-10b abrogated miR-10b actions on invasion. Second, siRNA-mediated silencing of TIP30 resulted in increased EGF-mediated invasiveness. Third, both high miR-10b levels and TIP30 knockdown enhanced EGF-induced EGFR tyrosine phosphorylation and ERK phosphorylation, whereas attenuating EGFR downregulation. Fourth, EGF-mediated invasion in the presence of high miR-10b levels was blocked by erlotinib and by the combined actions of the PI3K inhibitor LY294002 and the MEK inhibitor UO126, two key pathways downstream of EGFR activation.
PCCs and PDACs express high levels of EGFR, a known mediator of mitogenic signals in general and a promoter of PCC invasion and metastasis that is essential for PDAC initiation and progression.44, 45, 46 PDACs also express high levels of human EGFR 2 (HER2) and HER3, which heterodimerize with EGFR thereby diversifying and amplifying signaling cascades.47, 48 Moreover, PDACs express high levels of TGF-βs, 14-3-3sigma, MUC1, β1-integrins and delta-N p63, all of which enhance EGFR’s ability to activate aberrant pathways that contribute to the invasiveness of PCCs.26, 33, 49, 50, 51, 52 In the present study, we also determined that TGF-β increased EGF-mediated cell invasion and that this effect was markedly enhanced by high levels of miR-10b. Impressively, the combination of EGF, TGF-β and miR-10b induced a marked increase in cancer cell invasion and EMT-like alterations in gene expression. Thus, miR-10b was facilitating deleterious cross-talk between EGF and TGF-β in a manner that promotes PCC invasion. Given that miR-10b, EGFR and TGF-β are often overexpressed in PDAC,22, 23, 24, 25, 26 these observations suggest that suppressing miR-10b may prevent invasion and possibly metastasis in PDAC while interrupting deleterious EGF–TGF-β interactions that have the potential to contribute to PCC proliferation in vivo.
EGF binding to EGFR activates multiple signaling pathways in PCCs, such as the Ras/Raf/MAPK and Rac/JNK/MAPK-38,51 and EGF then undergoes rapid endocytosis.53 The EGF/EGFR complexes initially enter into the early endosomes where the EGFR kinase is still active54, 55 and traffic to the late endosome before entering the lysosome where EGFR undergoes degradation.56 TIP30 accelerates EGFR progression from early-to-late endosomes, whereas TIP30 silencing causes the retention of EGF–EGFR complexes in the early endosome thereby attenuating EGFR degradation and prolonging its signaling.31, 32, 57 Inasmuch as PDAC is associated with the overexpression of multiple members of the EGF family, including TGF-α, heparin-binding EGF-like growth factor, amphiregulin and betacellulin,44 our findings raise the possibility that loss of TIP30 in PDAC may lead to EGFR upregulation in the face of high levels of EGFR ligands that would otherwise promote EGFR degradation. Moreover, loss of TIP30 enhances susceptibility to tumorigenesis29 and has been associated with metastatic breast cancer.58 Taken together, these observations suggest that increased miR-10b in PDAC may promote PCC proliferation and metastasis and increase disease aggressiveness by suppressing TIP30 and upregulating EGFR. In support of this conclusion, lower TIP30 levels were associated with a more poorly differentiated histology, and T3M4 cells transduced to overexpress miR-10b exhibited accelerated PCC proliferation and intra-pancreatic tumor growth.
miR-10b promotes invasion in several cancer types by suppressing the expression of various suppressors of metastasis. In breast cancer, in addition to targeting HOXD10, miR-10b enhances invasion by targeting syndecan-1 (SDC1), which is known to suppress metastases.39 Moreover, miR-10b may act by suppressing KLF4, a zinc finger transcription factor which can also suppress metastasis formation,37, 59 and NF-1,40 which attenuates ras activity. In the present study, miR-10b did not alter HOXD10 or TIAM-1 expression, but it decreased the expression of SDC1, NF1 and KLF4. This selective targeting suggests that additional factors may bind to the non-targeted mRNA moieties in PCCs, rendering their sites inaccessible to miR-10b, as has been reported for miR-34a.60
Gene profiling identified two new potential targets downregulated by miR-10b, RAP2A and EPHB2. Bioinformatic analysis predicted that both genes harbor at least one miR-10b binding site, suggesting that they are direct targets of miR-10b. RAP2A has been implicated in cell adhesion and is induced by LKB1, whose loss of function is associated with altered cell polarity and enhanced metastases in lung cancer.61, 62 Similarly, loss of EPHB2 has been associated with increased metastasis in colorectal and gastric cancer.63, 64 miR-10b also enhanced EGF and TGF-β-induced expression of EMT-associated genes decreasing E-cadherin levels, whereas increasing N-cadherin, vimentin, Twist, Snail and Zeb1 levels. Together, these findings suggest that miR-10b modulates a repertoire of genes in PCCs whose downregulation enhances the metastatic process while promoting EMT, which further increases the motility and metastatic potential of these cells.
A number of reports have pointed to reciprocal modulatory interactions between specific miRs and members of the EGF receptor family. Thus, EGFR activation leads to increased expression of miR-21,65 miR-221 and miR-222.66 Conversely, miR-7 and miR-133b suppress EGFR expression,67, 68, 69 whereas miR-125a and 125b suppress HER2/HER3, and miR-205 targets HER3 but not other HERs.70, 71 To our knowledge, this is the first study showing the potential connection between miR-10b and EGFR signaling.
Our findings also demonstrate that miR-10b facilitates potentially deleterious interactions between EGFR and TGF-β pathways. Although the exact mechanisms whereby these interactions are enhanced by miR-10b are not known, there are several potentially complementary possibilities. First, TGF-β receptors undergo endocytosis and recycling, and their enhanced retention in the early endosome can increase TGF-β receptor signaling.72 It is therefore possible that co-retention in the early endosome may facilitate cross-talks between these receptors. Second, both MAPK activation and Smad signaling are essential for TGF-β-induced EMT,73 and miR-10b enhanced EGF’s ability to activate MAPK. Third, within the endosome, EGFR induces AGO2 phosphorylation to AGO2-Y393, thereby reducing AGO2 binding to Dicer and attenuating the processing of precursor miRNAs to mature miRNAs.74, 75 By contrast, TGF-β promotes Drosha-mediated miRNA maturation76, 77 and enhances the expression of EMT-inducing miRs.35 These observations raise the possibility that TGF-β may act, in part, by interfering with EGFR’s actions on Dicer.
In summary, our findings indicate that circulating miR-10b levels could serve as a diagnostic biomarker in PDAC and suggest that targeting miR-10b may represent an important therapeutic approach in this deadly cancer that could reverse the multiple deleterious actions of this oncomir.
Materials and methods
Cell culture
ASPC-1 and PANC-1 human PCCs were obtained from ATCC (Manassas, VA, USA), which validated their authenticity. COLO-357 and T3M4 human PCCs were a gift from Dr RS Metzger at the Duke University. Their authenticity was validated by chromosomal analysis. ASPC-1 cells were grown in RPMI 1640, and PANC-1 and COLO-357 cells were grown in Dulbecco’s modified Eagle’s medium. Media were supplemented with 5% fetal bovine serum, 100 U/ml penicillin and 100 μg/ml streptomycin (complete medium) at 37 °C in humidified 5% CO2 incubator. Serum-free medium was supplemented with 0.1% bovine serum albumin, 5 μg/ml apotransferrin and 5 ng/ml sodium selenite. EGF (Millipore, Billerica, MA, USA) and TGF-β (Genentech, Inc., South San Francisco, CA, USA) were added at the indicated concentrations.
RNA isolation and quantitation
Total RNA was extracted using TRIZOL reagent (Invitrogen, Carlsbad, CA, USA). Assaying of mature miRNAs was performed using the TaqMan miRNA qRT–PCR kit (Life Technologies, Gaithersburg, MD, USA) using RNU6B as an internal control. To assay mRNAs, RNA (500 ng) was reverse transcribed to cDNA with a high-capacity RNA-to-cDNA master mix, and qRT–PCR was performed with Power SYBR Green Master Mix (both from Life Technologies). Primers were designed using the Prime-BLAST online tool.
Transfection
Cells were transfected with 20–60 nM miR-10b precursor (known as pre-miR hsa-miR-10b precursor, sense: 5′-UACCCUGUAGAACCGAAUUUGUG-3′, Life Technologies) or a control precursor (known as pre-miR non-targeting negative control, sense: 5′-UGUACUGCUUACGAUUCGGTT-3′) at 50% confluency. Transfected cells were plated for MTT (3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide) assay (24 h post transfection) or transwell invasion assays (48 h post transfection). Non-targeting siRNA or siRNAs (ON-TARGETplus siRNA, Dharmacon, Lafayette, CO, USA) designed to target TIP30 were used to silence TIP30 in COLO-357 (60 nM) and PANC-1 (20 nM) cells. For TIP30 rescue experiments, PANC-1 cells were transfected with 20 nM miR precursor and 250 ng of pCMV-SPORT6-TIP30 cDNA and Lipofectamine 2000 (both from Invitrogen), which was used in all transfection studies.
Transduction
To stably overexpress miR-10b in PCCs, the MDH1-PGK-GFP microRNA-10b retroviral construct.28 Addgene plasmid No. 16070) or an empty MDH1-PGK-GFP construct (Addgene plasmid No. 11375) were transfected into Phoenix cells, a 293 T-based retroviral packaging cell line (Life Technologies). Viruses were harvested, and PCCs were transduced as described.78 After 48 h, GFP-positive cells PCCs were isolated (Flow Cytometry Facility, Indiana University School of Medicine, Indianapolis, IN, USA), re-plated and cultured as described above.
MTT, invasion and migration assays
MTT assays were performed in 96-well plates.49 miR-10b precursor and precursor control transfected cells (5 × 104) were plated in Matrigel pre-coated transwell chambers (BD Biosciences, San Jose, CA, USA), and invasion assays were performed as previously reported.49, 78 UO126 (1 μM) were from Alexis Biochemicals (San Diego, CA, USA). Erlotinib (2 μM; Genentech, Inc.) and LY294002 (10 μM; Calbiochem, San Diego, CA, USA) were added to serum-free medium in the lower chamber in the absence or presence of 1 nM EGF and/or 0.5 nM TGF-β1. Wound-healing assays were performed as previously reported49, 78 24 h after cell transfection with pre-miR-10b oligonucleotides.
Microarray and data analysis
miR-10b precursor or control transfected PANC-1 cells were harvested at 20 h post transfection. Total RNA (three replicates per condition) was extracted using TRIZOL reagent. Microarray analysis for microRNAs was performed by the Genomics and Microarray Core Facility at Dartmouth Medical School. Array data were registered with GEO (accession No. GSE40189) for public access.
Immunoblotting
Whole-cell extracts were prepared in ice-cold lysis buffer consisting of 20 mM Tris-HCl (pH 7.5), 150 mM NaCl, 1 mM EDTA, 1 mM EGTA (ethylene glycol tetraacetic acid), 1% Triton, 2.5 mM sodium pyrophosphate, 1 mM beta-glycerophosphate, 1 mM Na3VO4, 1 mM PMSF and one tablet of complete protease inhibitor/10 ml. Lysates (25 μg/lane) were subjected to 10-12% SDS-PAGE (sodium dodecyl sulfate-polyacrylamide gel electrophoresis) and transferred to PVDF (polyvinylidene difluoride; Millipore) and immunoblotted.33 Antibodies directed against the following antigens were used: EGFR, Phospho-EGFR Tyr1148, phospho-AKT Ser473, AKT, phospho-p44/42 MAPK Thr202/Tyr204, p44/42 MAPK, phospho-p38 MAPK Thr180/Tyr182, p38 MAPK (all from Cell Signaling Technology, Danvers, MA, USA), and ERK2 (C-14, Santa Cruz Biotechnology, Santa Cruz, CA, USA). The anti-TIP30 antibody was a kind gift from Dr Hua Xiao at the Michigan State University.
Constructs
The LightSwitch TIP30 3′UTR Reporter (Switchgear Genomics, Menlo Park, CA, USA) was utilized to assess whether there is a direct interaction between miR-10b and TIP30 3′UTR. Site-directed mutagenesis kits (Stratagene, La Jolla, CA, USA) were used to introduce a six-base pair mutation in the seed-binding site of TIP30 3′UTR. The pCMV-SPORT6 TIP30 cDNA construct used in the TIP30 rescue experiment was purchased from Open Biosystems, Huntsville, AL, USA.
Luciferase reporter assay
Cells in 24-well plates (50% confluency) were co-transfected with miR-10b precursor (20 nM) and TIP30 3′UTR Reporter (200 ng). Luciferase activity was measured 24 h later using the Dual-Glo luciferase assay system (Promega, Fitchburg, WI, USA).
Circulating miR-10b quantitation
Plasma from 20 normal, 5 chronic pancreatitis and 17 PDAC patients was obtained from the Indiana University Simon Cancer Center Solid Tissue Bank (Indianapolis, IN, USA). RNA was isolated from 100 μl of plasma using Trizol LS (Life Technologies, Carlsbad, CA, USA) according to the manufacturer’s recommendations. qRT–PCR for miR-10b was performed in duplicate with a Taqman micro RNA reverse transcription kit and a miR-10b-specific primer/probe set (Life Technologies). Ct values were obtained using a Viia 7 Real-time PCR System (Life Technologies). miR-10b levels were normalized to miR-16 levels, a stable and abundant circulating microRNA79 that is a suitable endogenous control for analyzing circulating microRNAs in PDAC plasma.80 Fold increases were calculated relative to miR-10b levels in normal plasma.
Orthotopic model
Sham- and miR-10b transduced T3M4 cells were injected into the tail region of each pancreas (500.000 cells/injection) of five 8-week-old male athymic mice. Tumors were imaged 3 weeks later with a Vevo2100 high resolution ultrasound (Visual Sonics Inc., Toronto, ON, Canada). Tumor volumes were calculated using the three-dimensional abdominal images and the Vevo2100 System software. Tumors were harvested, fixed, embedded, sectioned and hematoxylin and eosin stained.81
Immunohistochemistry
Paraffin-embedded human PDAC tissues were obtained from the Indiana University Simon Cancer Center Solid Tissue Bank, and 5-μm sections were stained for TIP30 using the anti-TIP30 antibody as described.82 For the orthotopic tumors, immunohistochemistry was performed on 5-μm sections using Ki67 (Novocastra, UK) and phospho-Histone H3 (Cell Signaling Technology, Inc.) antibodies.81, 82
Statistical analysis
Results are presented as mean±s.e.m. Statistical differences between groups were assessed using one sample t-test analysis, one-way or two-way ANOVA, as indicated, followed by Bonferroni post-test analysis. P<0.05 was considered as statistically significant.
Accession codes
References
Siegel R, Naishadham D, Jemal A . Cancer statistics 2012 CA Cancer J Clin 2012; 62: 10–29.
Lim JE, Chien MW, Earle CC . Prognostic factors following curative resection for pancreatic adenocarcinoma: a population-based, linked database analysis of 396 patients. Ann Surg 2003; 237: 74–85.
Hezel AF, Kimmelman AC, Stanger BZ, Bardeesy N, Depinho RA . Genetics and biology of pancreatic ductal adenocarcinoma. Genes Dev 2006; 20: 1218–1249.
Korc M . Pancreatic cancer-associated stroma production. Am J Surg 2007; 194: S84–S86.
Olive KP, Jacobetz MA, Davidson CJ, Gopinathan A, McIntyre D, Honess D et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science 2009; 324: 1457–1461.
Jones S, Zhang X, Parsons DW, Lin JC, Leary RJ, Angenendt P et al. Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science 2008; 321: 1801–1806.
Biankin AV, Waddell N, Kassahn KS, Gingras MC, Muthuswamy LB, Johns AL et al. Pancreatic cancer genomes reveal aberrations in axon guidance pathway genes. Nature 2012; 49: 399–405.
Kolch W, Pitt A . Functional proteomics to dissect tyrosine kinase signalling pathways in cancer. Nat Rev Cancer 2010; 10: 618–629.
Wagner JP, Wolf-Yadlin A, Sevecka M, Grenier JK, Root DE, Lauffenburger DA et al. Receptor tyrosine kinases fall into distinct classes based on their inferred signaling networks. Sci Signal 2013; 6: ra58.
Kang JS, Liu C, Derynck R . New regulatory mechanisms of TGF-beta receptor function. Trends Cell Biol 2009; 19: 385–394.
Padua D, Massagué J . Roles of TGFbeta in metastasis. Cell Res 2009; 19: 89–102.
Rowland-Goldsmith MA, Maruyama H, Matsuda K, Idezawa T, Ralli M et al. Soluble type II transforming growth factor-beta receptor attenuates expression of metastasis-associated genes and suppresses pancreatic cancer cell metastasis. Mol Cancer Therap 2002; 1: 161–167.
Sempere LF, Gunn JR, Korc M . A novel three-dimensional culture system uncovers growth stimulatory actions by TGF-beta in pancreatic cancer cells. Cancer Biol Ther 2011; 12: 198–207.
Valencia-Sanchez MA, Liu J, Hannon GJ, Parker R . Control of translation and mRNA degradation by miRNAs and siRNAs. Genes Dev 2006; 20: 515–524.
Esquela-Kerscher A, Slack FJ . Oncomirs—microRNAs with a role in cancer. Nat Rev Cancer 2006; 6: 259–269.
Iorio MV, Croce CM . MicroRNAs in cancer: small molecules with a huge impact. J Clin Oncol 2009; 27: 5848–5856.
Greither T, Grochola LF, Udelnow A, Lautenschlager C, Wurl P, Taubert H . Elevated expression of microRNAs 155, 203, 210 and 222 in pancreatic tumors is associated with poorer survival. Int J Cancer 2010; 126: 73–80.
Bloomston M, Frankel WL, Petrocca F, Volinia S, Alder H, Hagan JP et al. MicroRNA expression patterns to differentiate pancreatic adenocarcinoma from normal pancreas and chronic pancreatitis. JAMA 2007; 297: 1901–1908.
Szafranska AE, Doleshal M, Edmunds HS, Gordon S, Luttges J, Munding JB et al. Analysis of microRNAs in pancreatic fine-needle aspirates can classify benign and malignant tissues. Clin Chem 2008; 54: 1716–1724.
Lee EJ, Gusev Y, Jiang J, Nuovo GJ, Lerner MR, Frankel WL et al. Expression profiling identifies microRNA signature in pancreatic cancer. Int J Cancer 2007; 120: 1046–1054.
du Rieu MC, Torrisani J, Selves J, Al Saati T, Souque A, Dufresne M et al. MicroRNA-21 is induced early in pancreatic ductal adenocarcinoma precursor lesions. Clin Chem 2010; 56: 603–612.
Preis M, Gardner TB, Gordon SR, Pipas JM, Mackenzie TA, Klein EE et al. MicroRNA-10b expression correlates with response to neoadjuvant therapy and survival in pancreatic ductal adenocarcinoma. Clin Cancer Res 2011; 17: 5812–5821.
Setoyama T, Zhang X, Natsugoe S, Calin GA . microRNA-10b: a new marker or the marker of pancreatic ductal adenocarcinoma? Clin Cancer Res 2011; 17: 5527–5529.
Nakata K et al. MicroRNA-10b is overexpressed in pancreatic cancer, promotes its invasiveness, and correlates with a poor prognosis. Surgery 2011; 150: 916–922.
Korc M, Chandrasekar B, Yamanaka Y, Friess H, Büchler M, Beger H.G . Overexpression of the epidermal growth factor receptor in human pancreatic cancer is associated with concomitant increases in the levels of epidermal growth factor and transforming growth factor alpha. J Clin Invest 1992; 90: 1352–1360.
Friess H, Yamanaka Y, Büchler M, Ebert M, Beger H.G, Gold L.I et al. Enhanced expression of transforming growth factor-beta isoforms in pancreatic cancer correlates with decreased survival. Gastroenterology 1993; 105: 1846–1856.
Ma L, Teruya-Feldstein J, Weinberg RA . Tumour invasion and metastasis initiated by microRNA-10b in breast cancer. Nature 2007; 449: 682–688.
Ma L et al. Therapeutic silencing of miR-10b inhibits metastasis in a mouse mammary tumor model. Nat Biotechnol 2010; 28: 341–347.
Ito M, Jiang C, Krumm K, Zhang X, Pecha J, Zhao J et al. TIP30 deficiency increases susceptibility to tumorigenesis. Cancer Res 2003; 63: 8763–8767.
Tong X, Li K, Luo Z, Lu B, Liu X, Wang T et al. Decreased TIP30 expression promotes tumor metastasis in lung cancer. Am J Pathol 2009; 174: 1931–1939.
Zhang C, Mori M, Gao S, Li A, Hoshino I, Aupperlee MD et al. Tip30 deletion in MMTV-neu mice leads to enhanced EGFR signaling and development of estrogen receptor-positive and progesterone receptor-negative mammary tumors. Cancer Res 2010; 70: 10224–10233.
Zhang C, Li A, Zhang X, Xiao H . A novel TIP30 protein complex regulates EGF receptor signaling and endocytic degradation. J Biol Chem 2011; 286: 9373–9381.
Preis M, Korc M . Signaling pathways in pancreatic cancer. Crit Rev Eukaryot Gene Expr 2011; 21: 115–129.
Fang Y, Shi C, Manduchi E, Civelek M, Davies PF . MicroRNA-10a regulation of proinflammatory phenotype in athero-susceptible endothelium in vivo and in vitro. Proc Natl Acad Sci USA 2010; 107: 13450–13455.
Katsuno Y, Lamouille S, Derynck R . TGF-β signaling and epithelial–mesenchymal transition in cancer progression. Curr Opin Oncol 2013; 25: 76–84.
Moriarty CH, Pursell B, Mercurio AM . miR-10b targets Tiam1: implications for rac activation and carcinoma migration. J Biol Chem 2010; 285: 20541–20546.
Tian Y, Luo A, Cai Y, Su Q, Ding F, Chen H et al. MicroRNA-10b promotes migration and invasion through KLF4 in human esophageal cancer cell lines. J Biol Chem 2010; 285: 7986–7994.
Lin J, Teo S, Lam DH, Jeyaseelan K, Wang S . MicroRNA-10b pleiotropically regulates invasion, angiogenicity and apoptosis of tumor cells resembling mesenchymal subtype of glioblastoma multiforme. Cell Death Dis 2012; 3: e398.
Ibrahim SA, Yip GW, Stock C, Pan JW, Neubauer C, Poeter M et al. Targeting of syndecan-1 by microRNA miR-10b promotes breast cancer cell motility and invasiveness via a Rho-GTPase- and E-cadherin-dependent mechanism. Int J Cancer 2012; 131: E884–E896.
Chai G, Liu N, Ma J, Li H, Oblinger JL, Prahalad AK et al. MicroRNA-10b regulates tumorigenesis in neurofibromatosis type 1. Cancer Sci 2010; 101: 1997–2004.
Korc M, Meltzer P, Trent J . Enhanced expression of epidermal growth factor receptor correlates with alterations of chromosome 7 in human pancreatic cancer. Proc Natl Acad Sci USA 1986; 83: 5141–5144.
Nishida N, Yamashita S, Mimori K, Sudo T, Tanaka F, Shibata K et al2012. MicroRNA-10b is a prognostic indicator in colorectal cancer and confers resistance to the chemotherapeutic agent 5-fluorouracil in colorectal cancer cells. Ann Surg Oncol 19: 3065–3071.
Li Q-j Zhou L, Yang F, Wang G-X, Zheng H, Wang D-S et al2012. MicroRNA-10b promotes migration and invasion through CADM1 in human hepatocellular carcinoma cells. Tumor Biol 33: 1455–1465.
Korc M . Role of growth factors in pancreatic cancer. Surg Oncol Clin N Am 1998; 7: 25–41.
Ardito CM, Grüner BM, Takeuchi KK, Lubeseder-Martellato C, Teichmann N, Mazur PK et al. EGF receptor is required for KRAS-induced pancreatic tumorigenesis. Cancer Cell 2012; 22: 304–317.
Navas C, Hernández-Porras I, Schuhmacher AJ, Sibilia M, Guerra C, Barbacid M . EGF receptor signaling is essential for k-ras oncogene-driven pancreatic ductal adenocarcinoma. Cancer Cell 2012; 22: 318–330.
Yamanaka Y, Friess H, Kobrin MS, Büchler M, Kunz J, Beger HG et al. Overexpression of HER2/neu oncogene in human pancreatic carcinoma. Hum Pathol 1993; 24: 1127–1134.
Friess H, Yamanaka Y, Kobrin MS, Do DA, Büchler MW, Korc M . Enhanced erB-3 expression in human pancreatic cancer correlates with tumor progression. Clin Cancer Res 1995; 11: 1413–1420.
Neupane D, Korc M . 14-3-3sigma modulates pancreatic cancer cell survival and invasiveness. Clin Cancer Res 2008; 14: 7614–7623.
Hinoda Y, Ikematsu Y, Horinochi M, Sato S, Yamamoto K, Nakano T et al. Increased expression of MUC1 in advanced pancreatic cancer. J Gastroenterol 2003; 38: 1162–1166.
Lau SK, Shields DJ, Murphy EA, Desgrosellier JS, Anand S, Huang M et al. EGFR medicated carcinoma cell metastasis mediated by integrin αvβ5 depends on activation of c Src and cleavage of MUC1. PLoS One 2012; 7: e36753.
Danilov AV, Neupane D, Nagaraja AS, Feofanova EV, Humphries LA, DiRenzo J et al. DeltaNp63alpha-mediated induction of epidermal growth factor receptor promotes pancreatic cancer cell growth and chemoresistance. PLoS One 2011; 6: e26815.
Matsuda K, Idezawa T, You XJ, Kothari NH, Fan H, Korc M . Multiple mitogenic pathways in pancreatic cancer cells are blocked by a truncated epidermal growth factor receptor. Cancer Res 2002; 62: 5611–5617.
Miaczynska M, Pelkmans L, Zerial M . Not just a sink: endosomes in control of signal transduction. Curr Opin Cell Biol 2004; 16: 400–406.
Sorkin A, Goh LK . Endocytosis and intracellular trafficking of ErbBs. Exp Cell Res 2009; 315: 683–696.
Maxfield FR, McGraw TE . Endocytic recycling. Nat Rev Mol Cell Biol 2004; 5: 121–132.
Li A, Zhang C, Gao S, Chen F, Yang C, Luo R et al. TIP30 loss enhances cytoplasmic and nuclear EGFR signaling and promotes lung adenocarcinogenesis in mice. Oncogene 2012; 32: 2273–2281.
Zhao J, Ni H, Ma Y, Dong L, Dai J, Zhao F et al. TIP30/CC3 expression in breast carcinoma: relation to metastasis, clinicopathologic parameters, and P53 expression. Human Pathol 2007; 38: 293–298.
Yori JL, Seachrist DD, Johnson E, Lozada KL, Abdul-Karim FW, Chodosh LA et al. Kruppel-like factor 4 inhibits tumorigenic progression and metastasis in a mouse model of breast cancer. Neoplasia 2011; 13: 601–610.
Leveille N, Elkon R, Davalos V, Manoharan V, Hollingworth D, Oude Vrielink J et al. Selective inhibition of microRNA accessibility by RBM38 is required for p53 activity. Nat Commun 2011; 2: 513–524.
Gloerich M, ten Klooster JP, Vliem MJ, Koorman T, Zwartkruis FJ, Clevers H et al. Rap2A links intestinal cell polarity to brush border formation. Nat Cell Biol 2012; 14: 793–801.
Ji H, Ramsey MR, Hayes DN, Fan C, McNamara K, Kozlowski P et al. LKB1 modulates lung cancer differentiation and metastasis. Nature 2007; 448: 807–810.
Senior PV, Zhang BX, Chan ST . Loss of cell-surface receptor EphB2 is important for the growth, migration, and invasiveness of a colon cancer cell line. Int J Colorectal Dis 2010; 25: 687–694.
Yu G, Gao Y, Ni C, Chen Y, Pan J, Wang X et al. Reduced expression of EphB2 is significantly associated with nodal metastasis in Chinese patients with gastric cancer. J Cancer Res Clin Oncol 2011; 137: 73–80.
Seike M, Goto A, Okano T, Bowman ED, Schetter AJ, Horikawa I et al. MiR-21 is an EGFR-regulated anti-apoptotic factor in lung cancer in never-smokers. Proc Natl Acad Sci USA 2009; 106: 12085–12090.
Teixeira AL, Gomes M, Medeiros R . EGFR signaling pathway and related-miRNAs in age-related diseases: the example of miR-221 and miR-222. Front Genet 2012; 3: 286–292.
Webster RJ, Giles KM, Price KJ, Zhang PM, Mattick JS, Leedman PJ . Regulation of epidermal growth factor receptor signaling in human cancer cells by microRNA-7. J Biol Chem 2009; 284: 5731–5741.
Chou YT, Lin HH, Lien YC, Wang YH, Hong CF, Kao YR et al. EGFR promotes lung tumorigenesis by activating miR-7 through a Ras/ERK/Myc pathway that targets the Ets2 transcriptional repressor ERF. Cancer Res 2010; 70: 8822–8831.
Liu L, Shao X, Gao W, Zhang Z, Liu P, Wang R et al. MicroRNA-133b inhibits the growth of non-small-cell lung cancer by targeting the epidermal growth factor receptor. FEBS J 2012; 279: 3800–3812.
Nishida N, Mimori K, Fabbri M, Yokobori T, Sudo T, Tanaka F et al. MicroRNA-125a-5p is an independent prognostic factor in gastric cancer and inhibits the proliferation of human gastric cancer cells in combination with trastuzumab. Clin Cancer Res 2011; 17: 2725–2733.
Iorio MV, Casalini P, Piovan C, Di Leva G, Merlo A, Triulzi T et al. microRNA-205 regulates HER3 in human breast cancer. Cancer Res 2009; 69: 2195–2200.
Luga V, McLean S, Le Roy C, O'Connor-McCourt M, Wrana JL, Di Guglielmo GM . The extracellular domain of the TGFbeta type II receptor regulates membrane raft partitioning. Biochem J 2009; 421: 119–131.
Davies M, Robinson M, Smith E, Huntley S, Prime S, Paterson I . Induction of an epithelial to mesenchymal transition in human immortal and malignant keratinocytes by TGF-beta1 involves MAPK, Smad and AP-1 signalling pathways. J Cell Biochem 2005; 95: 918–931.
Shen J, Xia W, Khotskaya YB, Huo L, Nakanishi K, Lim SO et al. EGFR modulates microRNA maturation in response to hypoxia through phosphorylation of AGO2. Nature 2013; 497: 383–387.
Nishida N, Mimori K, Mori M, Calin GA . EGFR gets in the way of microRNA biogenesis. Cell Res (epub ahead of print 9 July 2013; doi:10.1038/cr.2013.87).
Davis BN, Hilyard AC, Lagna G, Hata A . SMAD proteins control DROSHA-mediated microRNA maturation. Nature 2008; 454: 56–61.
Hata A, Davis BN . Regulation of pri-miRNA processing through Smads. Adv Exp Med Biol 2011; 700: 15–27.
Liu F, Korc M . Cdk4/6 inhibition induces epithelial mesenchymal transition and enhances invasiveness in pancreatic cancer cells. Mol Cancer Ther 2012; 11: 2138–2148.
Mitchell PS, Parkin RK, Kroh EM, Fritz BR, Wyman SK, Pogosova-Agadjanyan EL et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc Natl Acad Sci 2008; 105: 10513–10518.
Wang J, Chen J, Chang P, LeBlanc A, Li D, Abbruzzesse JL et al. MicroRNAs in plasma of pancreatic ductal adenocarcinoma patients as novel blood-based biomarkers of disease. Cancer Prev Res 2009; 2: 807–813.
Carrière C, Gore AJ, Norris AM, Gunn JR, Young AL, Longnecker DS et al. Deletion of Rb accelerates pancreatic carcinogenesis by oncogenic Kras and impairs senescence in premalignant lesions. Gastroenterology 2011; 141: 1091–1101.
Zhang H, Zhang Y, Duan HO, Kirley SD, Lin SX, McDougal WS et al. TIP30 is associated with progression and metastasis of prostate cancer. Int J Cancer 2008; 123: 810–816.
Acknowledgements
This work is dedicated to the fond memory of Victor Fung, Ph.D., a former Program Officer at NCI and former Scientific Review Officer of the Cancer Etiology study section of CSR, NIH, for his wisdom, compassion, integrity, his love of sciences and the arts, his incredible culinary skills, and above all, his contributions to the career development of so many investigators during his own distinguished career. The work was supported, in part, by an NIH grant R37-CA-075059 to M K. HO was a Ph.D. candidate in the Program in Experimental and Molecular Medicine at the Geisel School of Medicine at Dartmouth. We thank the IUSCC Cancer Center at Indiana University School of Medicine for the use of the Tissue Procurement and Distribution Core, which provided pancreatic tissues and patient plasma for microRNA assays. We also thank Dr Hua Xiao at the Michigan State University for generously providing the anti-TIP30 antibody.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Ouyang, H., Gore, J., Deitz, S. et al. microRNA-10b enhances pancreatic cancer cell invasion by suppressing TIP30 expression and promoting EGF and TGF-β actions. Oncogene 33, 4664–4674 (2014). https://doi.org/10.1038/onc.2013.405
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2013.405
Keywords
This article is cited by
-
Molecular and metabolic regulation of immunosuppression in metastatic pancreatic ductal adenocarcinoma
Molecular Cancer (2023)
-
Pancreatic cancer stemness: dynamic status in malignant progression
Journal of Experimental & Clinical Cancer Research (2023)
-
Targeting miRNA and using miRNA as potential therapeutic options to bypass resistance in pancreatic ductal adenocarcinoma
Cancer and Metastasis Reviews (2023)
-
Extracellular vesicle biomarkers for pancreatic cancer diagnosis: a systematic review and meta-analysis
BMC Cancer (2022)
-
Low miR-10b-3p associated with sorafenib resistance in hepatocellular carcinoma
British Journal of Cancer (2022)