Abstract
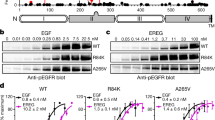
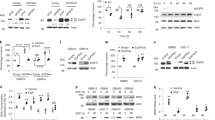
EGFRvIII is a key oncogene in glioblastoma (GBM). EGFRvIII results from an in-frame deletion in the extracellular domain of EGFR, does not bind ligand and is thought to be constitutively active. Although EGFRvIII dimerization is known to activate EGFRvIII, the factors that drive EGFRvIII dimerization and activation are not well understood. Here we present a new model of EGFRvIII activation and propose that oncogenic activation of EGFRvIII in glioma cells is driven by co-expressed activated EGFR wild type (EGFRwt). Increasing EGFRwt leads to a striking increase in EGFRvIII tyrosine phosphorylation and activation while silencing EGFRwt inhibits EGFRvIII activation. Both the dimerization arm and the kinase activity of EGFRwt are required for EGFRvIII activation. EGFRwt activates EGFRvIII by facilitating EGFRvIII dimerization. We have previously identified HB-EGF, a ligand for EGFRwt, as a gene induced specifically by EGFRvIII. In this study, we show that HB-EGF is induced by EGFRvIII only when EGFRwt is present. Remarkably, altering HB-EGF recapitulates the effect of EGFRwt on EGFRvIII activation. Thus, increasing HB-EGF leads to a striking increase in EGFRvIII tyrosine phosphorylation while silencing HB-EGF attenuates EGFRvIII phosphorylation, suggesting that an EGFRvIII–HB-EGF–EGFRwt feed-forward loop regulates EGFRvIII activation. Silencing EGFRwt or HB-EGF leads to a striking inhibition of EGFRvIII-induced tumorigenicity, while increasing EGFRwt or HB-EGF levels resulted in accelerated EGFRvIII-mediated oncogenicity in an orthotopic mouse model. Furthermore, we demonstrate the existence of this loop in human GBM. Thus, our data demonstrate that oncogenic activation of EGFRvIII in GBM is likely maintained by a continuous EGFRwt–EGFRvIII–HB-EGF loop, potentially an attractive target for therapeutic intervention.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Reardon DA, Wen PY . Therapeutic advances in the treatment of glioblastoma: rationale and potential role of targeted agents. Oncologist 2006; 11: 152–164.
Verhaak RG, Hoadley KA, Purdom E, Wang V, Qi Y, Wilkerson MD et al. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell 2010; 17: 98–110.
Feng H, Hu B, Liu KW, Li Y, Lu X, Cheng T et al. Activation of Rac1 by Src-dependent phosphorylation of Dock180(Y1811) mediates PDGFRalpha-stimulated glioma tumorigenesis in mice and humans. J Clin Invest 2011; 121: 4670–4684.
Libermann TA, Nusbaum HR, Razon N, Kris R, Lax I, Soreq H et al. Amplification, enhanced expression and possible rearrangement of EGF receptor gene in primary human brain tumours of glial origin. Nature 1985; 313: 144–147.
Huang PH, Xu AM, White FM . Oncogenic EGFR signaling networks in glioma. Sci Signal 2009; 2: re6.
Mellinghoff IK, Wang MY, Vivanco I, Haas-Kogan DA, Zhu S, Dia EQ et al. Molecular determinants of the response of glioblastomas to EGFR kinase inhibitors. N Engl J Med 2005; 353: 2012–2024.
Barkovich KJ, Hariono S, Garske AL, Zhang J, Blair JA, Fan QW et al. Kinetics of inhibitor cycling underlie therapeutic disparities between EGFR-driven lung and brain cancers. Cancer Discov 2012; 2: 450–457.
Vivanco I, Robins HI, Rohle D, Campos C, Grommes C, Nghiemphu PL et al. Differential sensitivity of glioma- versus lung cancer-specific EGFR mutations to EGFR kinase inhibitors. Cancer Discov 2012; 2: 458–471.
Nishikawa R, Ji XD, Harmon RC, Lazar CS, Gill GN, Cavenee WK et al. A mutant epidermal growth factor receptor common in human glioma confers enhanced tumorigenicity. Proc Natl Acad Sci USA 1994; 91: 7727–7731.
Nagane M, Coufal F, Lin H, Bogler O, Cavenee WK, Huang HJ . A common mutant epidermal growth factor receptor confers enhanced tumorigenicity on human glioblastoma cells by increasing proliferation and reducing apoptosis. Cancer Res 1996; 56: 5079–5086.
Holland EC, Hively WP, DePinho RA, Varmus HE . A constitutively active epidermal growth factor receptor cooperates with disruption of G1 cell-cycle arrest pathways to induce glioma-like lesions in mice. Genes Dev 1998; 12: 3675–3685.
Zhu H, Acquaviva J, Ramachandran P, Boskovitz A, Woolfenden S, Pfannl R et al. Oncogenic EGFR signaling cooperates with loss of tumor suppressor gene functions in gliomagenesis. Proc Natl Acad Sci USA 2009; 106: 2712–2716.
Chung I, Akita R, Vandlen R, Toomre D, Schlessinger J, Mellman I . Spatial control of EGF receptor activation by reversible dimerization on living cells. Nature 2010; 464: 783–787.
Moscatello DK, Montgomery RB, Sundareshan P, McDanel H, Wong MY, Wong AJ . Transformational and altered signal transduction by a naturally occurring mutant EGF receptor. Oncogene 1996; 13: 85–96.
Huang HS, Nagane M, Klingbeil CK, Lin H, Nishikawa R, Ji XD et al. The enhanced tumorigenic activity of a mutant epidermal growth factor receptor common in human cancers is mediated by threshold levels of constitutive tyrosine phosphorylation and unattenuated signaling. J Biol Chem 1997; 272: 2927–2935.
Luwor RB, Zhu HJ, Walker F, Vitali AA, Perera RM, Burgess AW et al. The tumor-specific de2-7 epidermal growth factor receptor (EGFR) promotes cells survival and heterodimerizes with the wild-type EGFR. Oncogene 2004; 23: 6095–6104.
Hwang Y, Chumbalkar V, Latha K, Bogler O . Forced dimerization increases the activity of DeltaEGFR/EGFRvIII and enhances its oncogenicity. Mol Cancer Res 2011; 9: 1199–1208.
Ramnarain DB, Park S, Lee DY, Hatanpaa KJ, Scoggin SO, Otu H et al. Differential gene expression analysis reveals generation of an autocrine loop by a mutant epidermal growth factor receptor in glioma cells. Cancer Res 2006; 66: 867–874.
Inda MD, Bonavia R, Mukasa A, Narita Y, Sah DW, Vandenberg S et al. Tumor heterogeneity is an active process maintained by a mutant EGFR-induced cytokine circuit in glioblastoma. Genes Dev 2010; 24: 1731–1745.
Dowlati A, Nethery D, Kern JA . Combined inhibition of epidermal growth factor receptor and JAK/STAT pathways results in greater growth inhibition in vitro than single agent therapy. Mol Cancer Ther 2004; 3: 459–463.
Thomas CY, Chouinard M, Cox M, Parsons S, Stallings-Mann M, Garcia R et al. Spontaneous activation and signaling by overexpressed epidermal growth factor receptors in glioblastoma cells. Int J Cancer 2003; 104: 19–27.
Schlessinger J . Cell signaling by receptor tyrosine kinases. Cell 2000; 103: 211–225.
Ferguson KM . Structure-based view of epidermal growth factor receptor regulation. Annu Rev Biophys 2008; 37: 353–373.
Wang Q, Villeneuve G, Wang Z . Control of epidermal growth factor receptor endocytosis by receptor dimerization, rather than receptor kinase activation. EMBO Rep 2005; 6: 942–948.
Garrett TP, McKern NM, Lou M, Elleman TC, Adams TE, Lovrecz GO et al. Crystal structure of a truncated epidermal growth factor receptor extracellular domain bound to transforming growth factor alpha. Cell 2002; 110: 763–773.
Magnus N, Garnier D, Rak J . Oncogenic epidermal growth factor receptor up-regulates multiple elements of the tissue factor signaling pathway in human glioma cells. Blood 2010; 116: 815–818.
Stommel JM, Kimmelman AC, Ying H, Nabioullin R, Ponugoti AH, Wiedemeyer R et al. Coactivation of receptor tyrosine kinases affects the response of tumor cells to targeted therapies. Science 2007; 318: 287–290.
Huang PH, Mukasa A, Bonavia R, Flynn RA, Brewer ZE, Cavenee WK et al. Quantitative analysis of EGFRvIII cellular signaling networks reveals a combinatorial therapeutic strategy for glioblastoma. Proc Natl Acad Sci USA 2007; 104: 12867–12872.
Pillay V, Allaf L, Wilding AL, Donoghue JF, Court NW, Greenall SA et al. The plasticity of oncogene addiction: implications for targeted therapies directed to receptor tyrosine kinases. Neoplasia 2009; 11: 448–458.
Acquaviva J, Jun HJ, Lessard J, Ruiz R, Zhu H, Donovan M et al. Chronic activation of wild-type epidermal growth factor receptor and loss of Cdkn2a cause mouse glioblastoma formation. Cancer Res 2011; 71: 7198–7206.
Johns TG, Perera RM, Vernes SC, Vitali AA, Cao DX, Cavenee WK et al. The efficacy of epidermal growth factor receptor-specific antibodies against glioma xenografts is influenced by receptor levels, activation status, and heterodimerization. Clin Cancer Res 2007; 13: 1911–1925.
Mishima K, Higashiyama S, Asai A, Yamaoka K, Nagashima Y, Taniguchi N et al. Heparin-binding epidermal growth factor-like growth factor stimulates mitogenic signaling and is highly expressed in human malignant gliomas. Acta Neuropathol (Berl) 1998; 96: 322–328.
Samuels V, Barrett JM, Bockman S, Pantazis CG, Allen MB Jr. . Immunocytochemical study of transforming growth factor expression in benign and malignant gliomas. Am J Pathol 1989; 134: 894–902.
Biernat W, Huang H, Yokoo H, Kleihues P, Ohgaki H . Predominant expression of mutant EGFR (EGFRvIII) is rare in primary glioblastomas. Brain Pathol 2004; 14: 131–136.
Hatanpaa KJ, Burma S, Zhao D, Habib AA . Epidermal growth factor receptor (EGFR) in glioma: Signal transduction, neuropathology, imaging and radioresistance. Neoplasia 2010; 12: 675–684.
Nishikawa R, Sugiyama T, Narita Y, Furnari F, Cavenee WK, Matsutani M . Immunohistochemical analysis of the mutant epidermal growth factor, deltaEGFR, in glioblastoma. Brain Tumor Pathol 2004; 21: 53–56.
Del Vecchio CA, Giacomini CP, Vogel H, Jensen KC, Florio T, Merlo A et al. EGFRvIII gene rearrangement is an early event in glioblastoma tumorigenesis and expression defines a hierarchy modulated by epigenetic mechanisms. Oncogene 2012; 32: 2670–2681.
Ramnarain DB, Paulmurugan R, Park S, Mickey BE, Asaithamby A, Saha D et al. RIP1 links inflammatory and growth factor signaling pathways by regulating expression of the EGFR. Cell Death Differ 2008; 15: 344–353.
Batra SK, Castelino-Prabhu S, Wikstrand CJ, Zhu X, Humphrey PA, Friedman HS et al. Epidermal growth factor ligand-independent, unregulated, cell-transforming potential of a naturally occurring human mutant EGFRvIII gene. Cell Growth Differ 1995; 6: 1251–1259.
Tang CK, Gong XQ, Moscatello DK, Wong AJ, Lippman ME . Epidermal growth factor receptor vIII enhances tumorigenicity in human breast cancer. Cancer Res 2000; 60: 3081–3087.
Estrada-Bernal A, Lawler SE, Nowicki MO, Ray Chaudhury A, Van Brocklyn JR . The role of sphingosine kinase-1 in EGFRvIII-regulated growth and survival of glioblastoma cells. J Neurooncol 2011; 102: 353–366.
Smith K, Gunaratnam L, Morley M, Franovic A, Mekhail K, Lee S . Silencing of epidermal growth factor receptor suppresses hypoxia-inducible factor-2-driven VHL-/- renal cancer. Cancer Res 2005; 65: 5221–5230.
Puliyappadamba VT, Chakraborty S, Chauncey SS, Li L, Hatanpaa KJ, Mickey B et al. Opposing effect of EGFRwt on EGFRvIII mediated NF-kappaB activation with RIP1 as a cell death switch. Cell Reports 2013; 4: 764–775.
Acknowledgements
We thank Dr Mien-Chie Hung (MD Anderson Cancer Center) for the EGFRwt-Myc plasmid and Dr James Van Brocklyn (Ohio State University) for GBM9 cells. This work was supported in part by NIH grant RO1NS062080 to AH and by RO1 CA139217 to DAB. SB is supported by grants from the National Institutes of Health (RO1 CA149461), National Aeronautics and Space Administration (NNX13AI13G) and the Cancer Prevention and Research Institute of Texas (RP100644).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Li, L., Chakraborty, S., Yang, CR. et al. An EGFR wild type–EGFRvIII–HB-EGF feed-forward loop regulates the activation of EGFRvIII. Oncogene 33, 4253–4264 (2014). https://doi.org/10.1038/onc.2013.400
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2013.400
Keywords
This article is cited by
-
Macrophage/microglia-derived IL-1β induces glioblastoma growth via the STAT3/NF-κB pathway
Human Cell (2022)
-
Epidermal growth factor receptor and EGFRvIII in glioblastoma: signaling pathways and targeted therapies
Oncogene (2018)
-
YAP and MRTF-A, transcriptional co-activators of RhoA-mediated gene expression, are critical for glioblastoma tumorigenicity
Oncogene (2018)
-
HBEGF promotes gliomagenesis in the context of Ink4a/Arf and Pten loss
Oncogene (2017)
-
A TNF–JNK–Axl–ERK signaling axis mediates primary resistance to EGFR inhibition in glioblastoma
Nature Neuroscience (2017)