Abstract
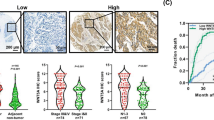
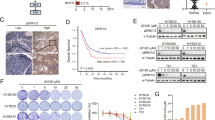
Signal transducer and activator of transcription 3 (STAT3) is altered in several epithelial cancers and represents a potential therapeutic target. Here, STAT3 expression, activity and cellular functions were examined in two main histotypes of esophageal carcinomas. In situ, immunohistochemistry for STAT3 and STAT3-Tyr705 phosphorylation (P-STAT3) in esophageal squamous cell carcinomas (ESCC, n=49) and Barrett’s adenocarcinomas (BAC, n=61) revealed similar STAT3 expression in ESCCs and BACs (P=0.109), but preferentially activated P-STAT3 in ESCCs (P=0.013). In vitro, strong STAT3 activation was seen by epidermal growth factor (EGF) stimulation in OE21 (ESCC) cells, whereas OE33 (BAC) cells showed constitutive weak STAT3 activation. STAT3 knockdown significantly reduced cell proliferation of OE21 (P=0.0148) and OE33 (P=0.0243) cells. Importantly, STAT3 knockdown reduced cell migration of OE33 cells by 2.5-fold in two types of migration assays (P=0.073, P=0.015), but not in OE21 cells (P=0.1079, P=0.386). Investigation of transcriptome analysis of STAT3 knockdown revealed a reduced STAT3 level associated with significant downregulation of cell cycle genes in both OE21 (P<0.0001) and OE33 (P=0.01) cells. In contrast, genes promoting cell migration (CTHRC1) were markedly upregulated in OE21 cells, whereas a gene linked to tight-junction stabilization and restricted cell motility (SHROOM2) was downregulated in OE21 but upregulated in OE33 cells. This study shows frequent, but distinct, patterns of STAT3 expression and activation in ESCCs and BACs. STAT3 knockdown reduces cell proliferation in ESCC and BAC cells, inhibits migration of BAC cells and may support cell migration of ESCC cells. Thereby, novel STAT3-regulated genes involved in ESCC and BAC cell proliferation and cell migration were identified. Thus, STAT3 may be further exploited as a potential novel therapeutic target, however, by careful distinction between the two histotypes of esophageal cancers.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Jove R . Preface: STAT signaling. Oncogene 2000; 19: 2466–2467.
Bromberg J . Stat proteins and oncogenesis. J Clin Invest 2002; 109: 1139–1142.
Vinkemeier U . Getting the message across, STAT! design principles of a molecular signaling circuit. J Cell Biol 2004; 167: 197–201.
Yu H, Pardoll D, Jove R . STATs in cancer inflammation and immunity: a leading role for STAT3. Nat Rev Cancer 2009; 9: 798–809.
Aggarwal BB, Kunnumakkara AB, Harikumar KB, Gupta SR, Tharakan ST, Koca C et al. Signal transducer and activator of transcription-3, inflammation, and cancer: how intimate is the relationship? Ann NY Acad Sci 2009; 1171: 59–76.
Sansone P, Bromberg J . Targeting the interleukin-6/Jak/stat pathway in human malignancies. J Clin Oncol 2012; 30: 1005–1014.
Haura EB, Zheng Z, Song L, Cantor A, Bepler G . Activated epidermal growth factor receptor-Stat-3 signaling promotes tumor survival in vivo in non-small cell lung cancer. Clin Cancer Res 2005; 11: 8288–8294.
Jiang R, Jin Z, Liu Z, Sun L, Wang L, Li K . Correlation of activated STAT3 expression with clinicopathologic features in lung adenocarcinoma and squamous cell carcinoma. Mol Diagn Ther 2011; 15: 347–352.
Seethala RR, Gooding WE, Handler PN, Collins B, Zhang Q, Siegfried JM, Grandis JR . Immunohistochemical analysis of phosphotyrosine signal transducer and activator of transcription 3 and epidermal growth factor receptor autocrine signaling pathways in head and neck cancers and metastatic lymph nodes. Clin Cancer Res 2008; 14: 1303–1309.
Gong W, Wang L, Yao JC, Ajani JA, Wei D, Aldape KD et al. Expression of activated signal transducer and activator of transcription 3 predicts expression of vascular endothelial growth factor in and angiogenic phenotype of human gastric cancer. Clin Cancer Res 2005; 11: 1386–1393.
Lee J, Kang WK, Park JO, Park SH, Park YS, Lim HY et al. Expression of activated signal transducer and activator of transcription 3 predicts poor clinical outcome in gastric adenocarcinoma. APMIS 2009; 117: 598–606.
Lassmann S, Schuster I, Walch A, Göbel H, Jütting U, Makowiec F et al. STAT3 mRNA and protein expression in colorectal cancer: effects on STAT3-inducible targets linked to cell survival and proliferation. J Clin Pathol 2007; 60: 173–179.
Morikawa T, Baba Y, Yamauchi M, Kuchiba A, Nosho K, Shima K et al. STAT3 expression, molecular features, inflammation patterns, and prognosis in a database of 724 colorectal cancers. Clin Cancer Res 2011; 17: 1452–1462.
Zhao W, Jaganathan S, Turkson J . A cell-permeable Stat3 SH2 domain mimetic inhibits Stat3 activation and induces antitumor cell effects in vitro. J Biol Chem 2010; 285: 35855–35865.
Leong PL, Andrews GA, Johnson DE, Dyer KF, Xi S, Mai JC et al. Targeted inhibition of Stat3 with a decoy oligonucleotide abrogates head and neck cancer cell growth. Proc Natl Acad Sci USA 2003; 100: 4138–4143.
Souissi I, Najjar I, Ah-Koon L, Schischmanoff PO, Lesage D, Le Coquil S et al. A STAT3-decoy oligonucleotide induces cell death in a human colorectal carcinoma cell line by blocking nuclear transfer of STAT3 and STAT3-bound NF-κB. BMC Cell Biol 2011; 12: 14.
Boehm AL, Sen M, Seethala R, Gooding WE, Freilino M, Wong SM et al. Combined targeting of epidermal growth factor receptor, signal transducer and activator of transcription-3, and Bcl-X(L) enhances antitumor effects in squamous cell carcinoma of the head and neck. Mol Pharmacol 2008; 73: 1632–1642.
Bonner JA, Yang ES, Trummell HQ, Nowsheen S, Willey CD, Raisch KP . Inhibition of STAT-3 results in greater cetuximab sensitivity in head and neck squamous cell carcinoma. Radiother Oncol 2011; 99: 339–343.
Jaganathan S, Yue P, Turkson J . Enhanced sensitivity of pancreatic cancer cells to concurrent inhibition of aberrant signal transducer and activator of transcription 3 and epidermal growth factor receptor or Src. J Pharmacol Exp Ther 2010; 333: 373–381.
Nagaraj NS, Washington MK, Merchant NB . Combined blockade of Src kinase and epidermal growth factor receptor with gemcitabine overcomes STAT3-mediated resistance of inhibition of pancreatic tumor growth. Clin Cancer Res 2011; 17: 483–493.
Gabbert HE, Nakamura Y, Shimoda T, Field YK, Hainaut P, Inoue H . Esophageal squamous cell carcinomas. In: Bosman FT, Carneiro F, Hruban RH, Theise ND (eds.) WHO Classification of Tumours. Volume 3. (IARC Press, Lyon, France).
Werner M, Lambert R, Flejou JF, Keller G, Hainaut P, Stein HJ, Höfler H . Adenocarcinoma of the esophagus. In: Bosman FT, Carneiro F, Hruban RH, Theise ND (eds.) WHO Classification of Tumours. Volume 3. (IARC Press, Lyon, France).
Pennathur A, Gibson MK, Jobe BA, Luketich JD . Oesophageal carcinoma. Lancet 2013; 381: 400–412.
Andl CD, Mizushima T, Oyama K, Bowser M, Nakagawa H, Rustgi AK . EGFR-induced cell migration is mediated predominantly by the JAK-STAT pathway in primary esophageal keratinocytes. Am J Physiol Gastrointest Liver Physiol 2004; 287: G1227–G1237.
Dvorak K, Chavarria M, Payne CM, Ramsey L, Crowley-Weber C, Dvorakova B et al. Activation of the interleukin-6/STAT3 antiapoptotic pathway in esophageal cells by bile acids and low pH: relevance to barrett's esophagus. Clin Cancer Res 2007; 13: 5305–5313.
Zhang HY, Zhang Q, Zhang X, Yu C, Huo X, Cheng E et al. Cancer-related inflammation and Barrett's carcinogenesis: interleukin-6 and STAT3 mediate apoptotic resistance in transformed Barrett's cells. Am J Physiol Gastrointest Liver Physiol 2011; 300: G454–G460.
Zhang Y, Du XL, Wang CJ, Lin DC, Ruan X, Feng YB et al. Reciprocal activation between plk1 and stat3 contributes to survival and proliferation of esophageal cancer cells. Gastroenterology 2011; 142: 521–530.
Yan S, Zhou C, Zhang W, Zhang G, Zhao X, Yang S et al. beta-Catenin/TCF pathway upregulates STAT3 expression in human esophageal squamous cell carcinoma. Cancer Lett 2008; 271: 85–97.
Reiser J, Adair B, Reinheckel T . Specialized roles for cysteine cathepsins in health and disease. J Clin Invest 2010; 120: 3421–3431.
Kreuzaler PA, Staniszewska AD, Li W, Omidvar N, Kedjouar B, Turkson J et al. Stat3 controls lysosomal-mediated cell death in vivo. Nat Cell Biol 2011; 13: 303–309.
Boonstra JJ, van Marion R, Beer DG, Lin L, Chaves P, Ribeiro C et al. Verification and unmasking of widely used human esophageal adenocarcinoma cell lines. J Natl Cancer Inst 2010; 102: 271–274.
Fichter CD, Herz C, Münch C, Opitz OG, Werner M, Lassmann S . Occurrence of multipolar mitoses and association with Aurora-A/-B kinases and p53 mutations in aneuploid esophageal carcinoma cells. BMC Cell Biol 2011; 12: 13.
Herz C, Schlürmann F, Batarello D, Fichter CD, Schöpflin A, Münch C et al. Occurrence of Aurora A positive multipolar mitoses in distinct molecular classes of colorectal carcinomas and effect of Aurora A inhibition. Mol Carcinog 2012; 51: 696–710.
Luo W, Friedman MS, Shedden K, Hankenson KD, Woolf PJ . GAGE: generally applicable gene set enrichment for pathway analysis. BMC Bioinformatics 2009; 27 (10): 161.
Park EH, Kim S, Jo JY, Kim SJ, Hwang Y, Kim JM et al. Collagen triple helix repeat containing-1 promotes pancreatic cancer progression by regulating migration and adhesion of tumor cells. Carcinogenesis 2013; 34: 694–702.
Etournay R, Zwaenepoel I, Perfettini I, Legrain P, Petit C, El-Amraoui A . Shroom2, a myosin-VIIa- and actin-binding protein, directly interacts with ZO-1 at tight junctions. J Cell Sci 2007; 120: 2838–2850.
Farber MJ, Rizaldy R, Hildebrand JD . Shroom2 regulates contractility to control endothelial morphogenesis. Mol Biol Cell 2011; 22: 795–805.
Hanawa M, Suzuki S, Dobashi Y, Yamane T, Kono K, Enomoto N, Ooi A . EGFR protein overexpression and gene amplification in squamous cell carcinomas of the esophagus. Int J Cancer 2006; 118: 1173–1180.
Yu WW, Guo YM, Zhu M, Cai XW, Zhu ZF, Zhao WX, Fu XL . Clinicopathological and prognostic significance of EGFR over-expression in esophageal squamous cell carcinoma: a meta-analysis. Hepatogastroenterology 2011; 58: 426–431.
Rauser S, Weis R, Braselmann H, Feith M, Stein HJ, Langer R et al. Significance of HER2 low-level copy gain in Barrett's cancer: implications for fluorescence in situ hybridization testing in tissues. Clin Cancer Res 2007; 13: 5115–5123.
Yoon HH, Shi Q, Sukov WR, Wiktor AE, Khan M, Sattler CA et al. Association of HER2/ErbB2 expression and gene amplification with pathologic features and prognosis in esophageal adenocarcinomas. Clin Cancer Res 2012; 18: 546–554.
Reipschlaeger S, Kubatzky K, Taromi S, Burger M, Orth J, Aktories K, Schmidt G . Toxin-induced RhoA activity mediates CCL1-triggered STAT signaling. J Biol Chem 2012; 287: 11183–11194.
Tischoff I, Hengge UR, Vieth M, Ell C, Stolte M, Weber A, Schmidt WE, Tannapfel A . Methylation of SOCS-3 and SOCS-1 in the carcinogenesis of Barrett's adenocarcinoma. Gut 2007; 56: 1047–1053.
Sobin LH, Gospodarowicz MK, Wittekind C (eds) TNM Classification of Malignant Tumours 7th Edn Wiley-Blackwell, UICC, Geneva, Switzerland, 2009).
Khoury JD, Medeiros LJ, Rassidakis GZ, Yared MA, Tsioli P, Leventaki V et al. Differential expression and clinical significance of tyrosine-phosphorylated STAT3 in ALK+ and ALK− anaplastic large cell lymphoma. Clin Cancer Res 2003; 9: 3692–3699.
Nasr MR, Laver JH, Chang M, Hutchison RE . Expression of anaplastic lymphoma kinase, tyrosine-phosphorylated STAT3, and associated factors in pediatric anaplastic large cell lymphoma: A report from the children's oncology group. Am J Clin Pathol 2007; 127: 770–778.
Gerlach U, Kayser G, Walch A, Hopt U, Schulte-Mönting J, Werner M, Lassmann S . Centrosome-, chromosomal-passenger- and cell-cycle-associated mRNAs are differentially regulated in the development of sporadic colorectal cancer. J Pathol 2006; 208: 462–472.
Lassmann S, Danciu M, Müller M, Weis R, Makowiec F, Schulte-Mönting J et al. Aurora A is differentially expressed and regulated in chromosomal and microsatellite instable sporadic colorectal cancers. Mod Pathol 2009; 22: 1385–1397.
Lassmann S, Kreutz C, Schoepflin A, Hopt U, Timmer J, Werner M . A novel approach for reliable microarray analysis of microdissected tumor cells from formalin-fixed and paraffin-embedded colorectal cancer resection specimens. J Mol Med 2009; 87: 211–224.
Livak KJ, Schmittgen TD . Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 2001; 25: 402–408.
Kamburov A, Stelzl U, Lehrach H, Herwig R . The ConsensusPathDB interaction database: 2013 update. Nucleic Acids Res 2013; 41: D793–D800.
Boerries M, Grahammer F, Eiselein S, Buck M, Meyer C, Goedel M et al. Molecular fingerprinting of the podocyte reveals novel gene and protein regulatory networks. Kidney Int 2013; 83: 1052–1064.
Acknowledgements
We thank Martina Gansz for assistance with Cathepsin analyses. We acknowledge the support of the study by the Deutsche Forschungsgemeinschaft (SFB850 project C5 to SL, MW; SFB850 project B7 to TR; Gerok stipend of SFB850 project Z1 to ST). Preliminary work for this study has been supported by the Mushett Family Foundation, Chester, NJ, USA (grant to SL, MW, LT, DK). TR and SL are member and associate members of BIOSS Centre for Biological Signalling Studies, Albert-Ludwigs-University, Freiburg, Germany. This study was supported by DFG SFB850 C5 (MW, SL); DFG SFB850 B7 (TR); DFG SFB850 Z1 (Gerok position to ST); and Mushett Family Foundation, Chester, NJ (DK, LT, MW, SL).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Timme, S., Ihde, S., Fichter, C. et al. STAT3 expression, activity and functional consequences of STAT3 inhibition in esophageal squamous cell carcinomas and Barrett’s adenocarcinomas. Oncogene 33, 3256–3266 (2014). https://doi.org/10.1038/onc.2013.298
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2013.298
Keywords
This article is cited by
-
The Critical Role of the Shroom Family Proteins in Morphogenesis, Organogenesis and Disease
Phenomics (2024)
-
TRIM29 hypermethylation drives esophageal cancer progression via suppression of ZNF750
Cell Death Discovery (2023)
-
PD-1 blockade enhances chemotherapy toxicity in oesophageal adenocarcinoma
Scientific Reports (2022)
-
Exosomal lncRNA ZFAS1 regulates esophageal squamous cell carcinoma cell proliferation, invasion, migration and apoptosis via microRNA-124/STAT3 axis
Journal of Experimental & Clinical Cancer Research (2019)
-
SHROOM2 inhibits tumor metastasis through RhoA–ROCK pathway-dependent and -independent mechanisms in nasopharyngeal carcinoma
Cell Death & Disease (2019)