Abstract
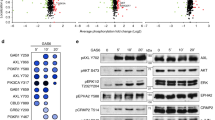
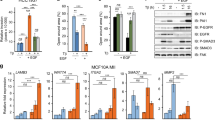
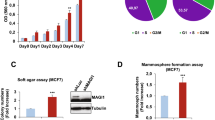
Krüppel-like factor 8 (KLF8) regulates critical gene transcription associated with cancer. The underlying mechanisms, however, remain largely unidentified. We have recently demonstrated that KLF8 expression enhances the activity but not expression of matrix metalloproteinase-2 (MMP2), the target substrate of MMP14. Here, we report a novel KLF8 to MMP14 signaling that promotes human breast cancer invasion and metastasis. Using cell lines for inducible expression and knockdown of KLF8, we demonstrate that KLF8 promotes MMP14 expression at the transcriptional level. Knocking down KLF8 expression inhibited the breast cancer cell invasion both in vitro and in vivo as well as the lung metastasis in mice, which could be rescued by ectopic expression of MMP14. Promoter reporter assays and oligonucleotide and chromatin immunoprecipitations determined that KLF8 activates the human MMP14 gene promoter by both directly acting on the promoter and indirectly via promoting the nuclear translocation of β-catenin, the expression of T-cell factor-1 (TCF1) and subsequent activation of the promoter by the β-catenin/TCF1 complex. Inhibition of focal adhesion kinase (FAK) using pharmacological inhibitor, RNA interference or knockout showed that the cell surface presentation of active MMP14 downstream of KLF8 depends on FAK expression and activity. Taken together, this work identified novel signaling mechanisms by which KLF8 and FAK work together to promote the extracellular activity of MMP14 critical for breast cancer metastasis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Lahiri SK, Zhao J . Kruppel-like factor 8 emerges as an important regulator of cancer. Am J Transl Res 2012; 4: 357–363.
Lu H, Wang X, Urvalek AM, Li T, Xie H, Yu L et al. Transformation of human ovarian surface epithelial cells by Kruppel-like factor 8. Oncogene (e-pub ahead of print 10 December 2012; doi: 10.1038/onc.2012.545).
Lu H, Hu L, Li T, Lahiri S, Shen C, Wason MS et al. A novel role of Kruppel-like factor 8 in DNA repair in breast cancer cells. J Biol Chem 2012; 287: 43720–43729.
Wang X, Lu H, Urvalek AM, Li T, Yu L, Lamar J et al. KLF8 promotes human breast cancer cell invasion and metastasis by transcriptional activation of MMP9. Oncogene 2011; 30: 1901–1911.
Wang X, Urvalek AM, Liu J, Zhao J . Activation of KLF8 transcription by focal adhesion kinase in human ovarian epithelial and cancer cells. J Biol Chem 2008; 283: 13934–13942.
Wang X, Zheng M, Liu G, Xia W, McKeown-Longo PJ, Hung MC et al. Kruppel-like factor 8 induces epithelial to mesenchymal transition and epithelial cell invasion. Cancer Res 2007; 67: 7184–7193.
Wang X, Zhao J . KLF8 transcription factor participates in oncogenic transformation. Oncogene 2007; 26: 456–461.
Zhang X, Yue P, Page BD, Li T, Zhao W, Namanja AT et al. Orally bioavailable small-molecule inhibitor of transcription factor Stat3 regresses human breast and lung cancer xenografts. Proc Natl Acad Sci USA 2012; 109: 9623–9628.
Schnell O, Romagna A, Jaehnert I, Albrecht V, Eigenbrod S, Juerchott K et al. Kruppel-like factor 8 (KLF8) is expressed in gliomas of different WHO grades and is essential for tumor cell proliferation. PLoS ONE 2012; 7: e30429.
Urvalek AM, Lu H, Wang X, Li T, Yu L, Zhu J et al. Regulation of the oncoprotein KLF8 by a switch between acetylation and sumoylation. Am J Transl Res 2011; 3: 121–132.
Urvalek AM, Wang X, Lu H, Zhao J . KLF8 recruits the p300 and PCAF co-activators to its amino terminal activation domain to activate transcription. Cell Cycle 2010; 9: 601–611.
Wei H, Wang X, Gan B, Urvalek AM, Melkoumian ZK, Guan JL et al. Sumoylation delimits KLF8 transcriptional activity associated with the cell cycle regulation. J Biol Chem 2006; 281: 16664–16671.
Zhao J, Bian ZC, Yee K, Chen BP, Chien S, Guan JL . Identification of transcription factor KLF8 as a downstream target of focal adhesion kinase in its regulation of cyclin D1 and cell cycle progression. Mol Cell 2003; 11: 1503–1515.
van Vliet J, Turner J, Crossley M . Human Kruppel-like factor 8: a CACCC-box binding protein that associates with CtBP and represses transcription. Nucleic Acids Res 2000; 28: 1955–1962.
Zhao J, Guan JL . Signal transduction by focal adhesion kinase in cancer. Cancer Metastasis Rev 2009; 28: 35–49.
Mehta TS, Lu H, Wang X, Urvalek AM, Nguyen KH, Monzur F et al. A unique sequence in the N-terminal regulatory region controls the nuclear localization of KLF8 by cooperating with the C-terminal zinc-fingers. Cell Res 2009; 19: 1098–1109.
Afsar NA, Ufer M, Haenisch S, Remmler C, Mateen A, Usman A et al. Relationship of drug metabolizing enzyme genotype to plasma levels as well as myelotoxicity of cyclophosphamide in breast cancer patients. Eur J Clin Pharmacol 2012; 68: 389–395.
Lu H, Wang X, Li T, Urvalek AM, Yu L, Li J et al. Identification of poly (ADP-ribose) polymerase-1 (PARP-1) as a novel Kruppel-like factor 8-interacting and -regulating protein. J Biol Chem 2011; 286: 20335–20344.
Lohi J, Lehti K, Valtanen H, Parks WC, Keski-Oja J . Structural analysis and promoter characterization of the human membrane-type matrix metalloproteinase-1 (MT1-MMP) gene. Gene 2000; 242: 75–86.
Wu X, Gan B, Yoo Y, Guan JL . FAK-mediated src phosphorylation of endophilin A2 inhibits endocytosis of MT1-MMP and promotes ECM degradation. Dev Cell 2005; 9: 185–196.
Takahashi M, Tsunoda T, Seiki M, Nakamura Y, Furukawa Y . Identification of membrane-type matrix metalloproteinase-1 as a target of the beta-catenin/Tcf4 complex in human colorectal cancers. Oncogene 2002; 21: 5861–5867.
Sanchez-Tillo E, de Barrios O, Siles L, Cuatrecasas M, Castells A, Postigo A . beta-catenin/TCF4 complex induces the epithelial-to-mesenchymal transition (EMT)-activator ZEB1 to regulate tumor invasiveness. Proc Natl Acad Sci USA 2011; 108: 19204–19209.
Liu P, Yang J, Pei J, Pei D, Wilson MJ . Regulation of MT1-MMP activity by beta-catenin in MDCK non-cancer and HT1080 cancer cells. J Cell Physiol 2010; 225: 810–821.
Barbolina MV, Stack MS . Membrane type 1-matrix metalloproteinase: substrate diversity in pericellular proteolysis. Semin Cell Dev Biol 2008; 19: 24–33.
Covington MD, Burghardt RC, Parrish AR . Ischemia-induced cleavage of cadherins in NRK cells requires MT1-MMP (MMP-14). Am J Physiol Renal Physiol 2006; 290: F43–F51.
Dwivedi DJ, Pino G, Banh A, Nathu Z, Howchin D, Margetts P et al. Matrix metalloproteinase inhibitors suppress transforming growth factor-beta-induced subcapsular cataract formation. Am J Pathol 2006; 168: 69–79.
Symowicz J, Adley BP, Gleason KJ, Johnson JJ, Ghosh S, Fishman DA et al. Engagement of collagen-binding integrins promotes matrix metalloproteinase-9-dependent E-cadherin ectodomain shedding in ovarian carcinoma cells. Cancer Res 2007; 67: 2030–2039.
Nawrocki-Raby B, Gilles C, Polette M, Bruyneel E, Laronze JY, Bonnet N et al. Upregulation of MMPs by soluble E-cadherin in human lung tumor cells. Int J Cancer 2003; 105: 790–795.
Ara T, Deyama Y, Yoshimura Y, Higashino F, Shindoh M, Matsumoto A et al. Membrane type 1-matrix metalloproteinase expression is regulated by E-cadherin through the suppression of mitogen-activated protein kinase cascade. Cancer Lett 2000; 157: 115–121.
Cristofanilli M . Circulating tumor cells, disease progression, and survival in metastatic breast cancer. Semin Oncol 2006; 33 (3 Suppl 9): S9–14.
Zhao JH, Reiske H, Guan JL . Regulation of the cell cycle by focal adhesion kinase. J Cell Biol 1998; 143: 1997–2008.
Veeman MT, Slusarski DC, Kaykas A, Louie SH, Moon RT . Zebrafish prickle, a modulator of noncanonical Wnt/Fz signaling, regulates gastrulation movements. Curr Biol 2003; 13: 680–685.
Acknowledgements
We appreciate Dr Jouko Lohi of University of Helsinki for kindly providing the human MMP14 gene promoter reporter vectors. This work was supported by grants from NCI (CA132977) and Susan G Komen for Cure breast cancer foundation (KG090444 and KG080616) to JZ.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Lu, H., Hu, L., Yu, L. et al. KLF8 and FAK cooperatively enrich the active MMP14 on the cell surface required for the metastatic progression of breast cancer. Oncogene 33, 2909–2917 (2014). https://doi.org/10.1038/onc.2013.247
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2013.247
Keywords
This article is cited by
-
Extracellular vesicles derived from bone marrow mesenchymal stem cells loaded on magnetic nanoparticles delay the progression of diabetic osteoporosis via delivery of miR-150-5p
Cell Biology and Toxicology (2023)
-
Monocytic MDSC mobilization promotes tumor recurrence after liver transplantation via CXCL10/TLR4/MMP14 signaling
Cell Death & Disease (2021)
-
KLF8 overexpression promotes the growth of human lung cancer cells by promoting the expression of JMJD2A
Cancer Cell International (2019)
-
Matrilysin/MMP-7 Cleavage of Perlecan/HSPG2 Complexed with Semaphorin 3A Supports FAK-Mediated Stromal Invasion by Prostate Cancer Cells
Scientific Reports (2018)
-
For robust big data analyses: a collection of 150 important pro-metastatic genes
Chinese Journal of Cancer (2017)