Abstract
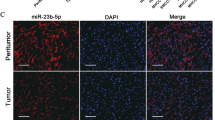
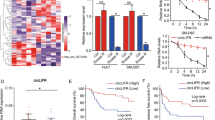
Mammalian sirtuin 1 (SIRT1) has connected to an ever widening circle of activities that encompass cellular stress resistance, energy metabolism and tumorigenesis. However, underlying mechanisms leading to oncogenic SIRT1 overexpression are less understood. In this study, we identified SIRT1 regulatory microRNA (miRNA) and its function in hepatocellular carcinoma (HCC). Aberrant SIRT1 overexpression was demonstrated in a subset of human HCCs. SIRT1 knockdown suppressed HCC cell growth by transcriptional deregulation of cell cycle proteins. This led to hypophosphorylation of pRb, which inactivated E2F/DP1 target gene transcription, and thereby caused significant increase of HCC cells to remain in the G1/S phase. A comprehensive miRNA profiling analysis indentified five putative endogenous miRNAs that are significantly downregulated in HCC. Ectopic expression of miRNA mimics evidenced miR-29c to suppress SIRT1 in HCC cells. Notably, ectopic miR-29c expression repressed cancer cell growth and proliferation, and it recapitulated SIRT1 knockdown effects in HCC cells. In addition, miR-29c expression was downregulated in a large cohort of HCC patients, and low expression of miR-29c was significantly associated with poor prognosis of HCC patients. Taken together, we demonstrated that miR-29c suppresses oncogenic SIRT1 by way of binding to 3′-untranslated region of SIRT1 mRNA causing translational inhibition in liver cancer cells. The loss or suppression of miR-29c may cause aberrant SIRT1 overexpression and promotes liver tumorigenesis. Overall, we suggest that miR-29c functions as a tumor suppressor by regulating abnormal SIRT1 activity in liver.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D . Global cancer statistics. CA Cancer J Clin 2011; 61: 69–90.
Whittaker S, Marais R, Zhu AX . The role of signaling pathways in the development and treatment of hepatocellular carcinoma. Oncogene 2010; 29: 4989–5005.
Fabbri M, Garzon R, Cimmino A, Liu Z, Zanesi N, Callegari E et al. MicroRNA-29 family reverts aberrant methylation in lung cancer by targeting DNA methyltransferases 3A and 3B. Proc Natl Acad Sci USA 2007; 104: 15805–15810.
Blander G, Guarente L . The Sir2 family of protein deacetylases. Annu Rev Biochem 2004; 73: 417–435.
Huffman DM, Grizzle WE, Bamman MM, Kim JS, Eltoum IA, Elgavish A et al. SIRT1 is significantly elevated in mouse and human prostate cancer. Cancer Res 2007; 67: 6612–6618.
Jang KY, Kim KS, Hwang SH, Kwon KS, Kim KR, Park HS et al. Expression and prognostic significance of SIRT1 in ovarian epithelial tumours. Pathology 2009; 41: 366–371.
Cha EJ, Noh SJ, Kwon KS, Kim CY, Park BH, Park HS et al. Expression of DBC1 and SIRT1 is associated with poor prognosis of gastric carcinoma. Clin Cancer Res 2009; 15: 4453–4459.
Stunkel W, Peh BK, Tan YC, Nayagam VM, Wang X, Salto-Tellez M et al. Function of the SIRT1 protein deacetylase in cancer. Biotechnol J 2007; 2: 1360–1368.
Chen J, Zhang B, Wong N, Lo AW, To KF, Chan AW et al. Sirtuin 1 is upregulated in a subset of hepatocellular carcinomas where it is essential for telomere maintenance and tumor cell growth. Cancer Res 2011; 71: 4138–4149.
Bartel DP . MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 2004; 116: 281–297.
Meng F, Henson R, Wehbe-Janek H, Ghoshal K, Jacob ST, Patel T . MicroRNA-21 regulates expression of the PTEN tumor suppressor gene in human hepatocellular cancer. Gastroenterology 2007; 133: 647–658.
Xiong Y, Fang JH, Yun JP, Yang J, Zhang Y, Jia WH et al. Effects of microRNA-29 on apoptosis, tumorigenicity, and prognosis of hepatocellular carcinoma. Hepatology 2010; 51: 836–845.
Pineau P, Volinia S, McJunkin K, Marchio A, Battiston C, Terris B et al. miR-221 overexpression contributes to liver tumorigenesis. Proc Natl Acad Sci USA 2010; 107: 264–269.
Ota H, Tokunaga E, Chang K, Hikasa M, Iijima K, Eto M et al. Sirt1 inhibitor, Sirtinol, induces senescence-like growth arrest with attenuated Ras-MAPK signaling in human cancer cells. Oncogene 2006; 25: 176–185.
Firestein R, Blander G, Michan S, Oberdoerffer P, Ogino S, Campbell J et al. The SIRT1 deacetylase suppresses intestinal tumorigenesis and colon cancer growth. PLoS One 2008; 3: e2020.
Wang RH, Sengupta K, Li C, Kim HS, Cao L, Xiao C et al. Impaired DNA damage response, genome instability, and tumorigenesis in SIRT1 mutant mice. Cancer Cell 2008; 14: 312–323.
Kabra N, Li Z, Chen L, Li B, Zhang X, Wang C et al. SirT1 is an inhibitor of proliferation and tumor formation in colon cancer. J Biol Chem 2009; 284: 18210–18217.
Noh JH, Jung KH, Kim JK, Eun JW, Bae HJ, Xie HJ et al. Aberrant regulation of HDAC2 mediates proliferation of hepatocellular carcinoma cells by deregulating expression of G1/S cell cycle proteins. PLoS One 6: e28103.
Xie HJ, Noh JH, Kim JK, Jung KH, Eun JW, Bae HJ et al. HDAC1 inactivation induces mitotic defect and caspase-independent autophagic cell death in liver cancer. PLoS One 7: e34265.
Saunders LR, Sharma AD, Tawney J, Nakagawa M, Okita K, Yamanaka S et al. miRNAs regulate SIRT1 expression during mouse embryonic stem cell differentiation and in adult mouse tissues. Aging (Albany NY) 2010; 2: 415–431.
Cao Q, Mani RS, Ateeq B, Dhanasekaran SM, Asangani IA, Prensner JR et al. Coordinated regulation of polycomb group complexes through microRNAs in cancer. Cancer Cell 2011; 20: 187–199.
Kriegel AJ, Liu Y, Fang Y, Ding X, Liang M . The miR-29 family: genomics, cell biology, and relevance to renal and cardiovascular injury. Physiol Genomics 2012; 44: 237–244.
Jung KH, Noh JH, Kim JK, Eun JW, Bae HJ, Chang YG et al. Histone deacetylase 6 functions as a tumor suppressor by activating c-Jun NH2-terminal kinase-mediated beclin 1-dependent autophagic cell death in liver cancer. Hepatology 2012; 56: 644–657.
Kim JK, Noh JH, Jung KH, Eun JW, Bae HJ, Kim MG et al. Sirtuin7 oncogenic potential in human hepatocellular carcinoma and its regulation by the tumor suppressors MiR-125a-5p and MiR-125b. Hepatology 2013; 57: 1055–1067.
Niyazi M, Niyazi I, Belka C . Counting colonies of clonogenic assays by using densitometric software. Radiat Oncol 2007; 2: 4.
Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (MEST; Grant No. 2011-0010705 and No. 2012M3A9D1054476), and by the Korean Science and Engineering Foundation via the ‘Cancer Evolution Research Center’ at the Catholic University of Korea.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Bae, H., Noh, J., Kim, J. et al. MicroRNA-29c functions as a tumor suppressor by direct targeting oncogenic SIRT1 in hepatocellular carcinoma. Oncogene 33, 2557–2567 (2014). https://doi.org/10.1038/onc.2013.216
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2013.216
Keywords
This article is cited by
-
miR-29a-SIRT1-Wnt/β-Catenin Axis Regulates Tumor Progression and Survival in Hepatocellular Carcinoma
Biochemical Genetics (2023)
-
Role of miR-653 and miR-29c in downregulation of CYP1A2 expression in hepatocellular carcinoma
Pharmacological Reports (2022)
-
Sirt1 antisense transcript is down-regulated in human tumors
Molecular Biology Reports (2019)
-
Differential expression of hsa-miR-221, hsa-miR-21, hsa-miR-135b, and hsa-miR-29c suggests a field effect in oral cancer
BMC Cancer (2018)
-
Regulation of SIRT1/AMPK axis is critically involved in gallotannin-induced senescence and impaired autophagy leading to cell death in hepatocellular carcinoma cells
Archives of Toxicology (2018)