Abstract
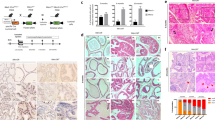
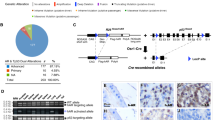
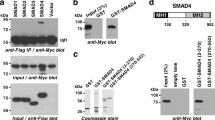
Multiple genetic alterations are associated with prostate carcinogenesis. Tumor-suppressor genes phosphatase and tensin homolog deleted on chromosome 10 (Pten) and androgen upregulated gene 19 (U19), which encodes ELL-associated factor 2 (EAF2), are frequently inactivated or downregulated in advanced prostate cancers. Previous studies showed that EAF2 knockout caused tumors in multiple organs and prostatic intraepithelial neoplasia (PIN) in mice. However, EAF2-knockout mice did not develop prostate cancer even at 2 years of age. To further define the roles of EAF2 in prostate carcinogenesis, we crossed the Pten+/− and EAF2+/− mice in the C57/BL6 background to generate EAF2−/−Pten+/−, Pten+/−, EAF2−/− and wild-type mice. The prostates from virgin male mice with the above four genotypes were analyzed at 7 weeks, 19 weeks and 12 months of age. Concomitant loss of EAF2 function and inactivation of one Pten allele induced spontaneous prostate cancer in 33% of the mice. Prostatic tissues from intact EAF2−/− Pten+/− mice exhibited higher levels of phospho-Akt, -p44/42 and microvessel density. Moreover, phospho-Akt remained high after castration. Consistently, there was a synergistic increase in prostate epithelial proliferation in both intact and castrated EAF2−/−Pten+/− mice. Using laser-capture microdissection coupled with real-time reverse transcription–PCR, we confirmed that co-downregulation of EAF2 and Pten occurred in >50% clinical prostate cancer specimens with Gleason scores of 8–9 (n=11), which is associated with poor prognosis. The above findings together demonstrated synergistic functional interactions and clinical relevance of concurrent EAF2 and Pten downregulation in prostate carcinogenesis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Jemal A, Siegel R, Xu J, Ward E . Cancer Statistics, 2010. CA Cancer J Clin 2010; 60: 277–300.
Shen MM, Abate-Shen C . Molecular genetics of prostate cancer: new prospects for old challenges. Genes Dev 2010; 24: 1967–2000.
Kim MJ, Cardiff RD, Desai N, Banach-Petrosky WA, Parsons R, Shen MM et al. Cooperativity of Nkx3.1 and Pten loss of function in a mouse model of prostate carcinogenesis. Proc Natl Acad Sci USA 2002; 99: 2884–2889.
Xiao W, Zhang Q, Jiang F, Pins M, Kozlowski JM, Wang Z . Suppression of prostate tumor growth by U19, a novel testosterone-regulated apoptosis inducer. Cancer Res 2003; 63: 4698–4704.
Xiao W, Zhang Q, Habermacher G, Yang X, Zhang AY, Cai X et al. U19/Eaf2 knockout causes lung adenocarcinoma, B-cell lymphoma, hepatocellular carcinoma and prostatic intraepithelial neoplasia. Oncogene 2008; 27: 1536–1544.
Eng C . PTEN: one gene, many syndromes. Hum Mutat 2003; 22: 183–198.
Bedolla R, Prihoda TJ, Kreisberg JI, Malik SN, Krishnegowda NK, Troyer DA et al. Determining risk of biochemical recurrence in prostate cancer by immunohistochemical detection of PTEN expression and Akt activation. Clin Cancer Res 2007; 13: 3860–3867.
Majumder PK, Sellers WR . Akt-regulated pathways in prostate cancer. Oncogene 2005; 24: 7465–7474.
Salmena L, Carracedo A, Pandolfi PP . Tenets of PTEN tumor suppression. Cell 2008; 133: 403–414.
Leslie NR, Downes CP . PTEN function: how normal cells control it and tumour cells lose it. Biochem J 2004; 382 (Pt 1): 1–11.
Hamada K, Sasaki T, Koni PA, Natsui M, Kishimoto H, Sasaki J et al. The PTEN/PI3K pathway governs normal vascular development and tumor angiogenesis. Genes Dev 2005; 19: 2054–2065.
Stiles B, Groszer M, Wang S, Jiao J, Wu H . PTENless means more. Dev Biol 2004; 273: 175–184.
Suzuki A, de la Pompa JL, Stambolic V, Elia AJ, Sasaki T, del Barco Barrantes I et al. High cancer susceptibility and embryonic lethality associated with mutation of the PTEN tumor suppressor gene in mice. Curr Biol 1998; 8: 1169–1178.
Di Cristofano A, Pesce B, Cordon-Cardo C, Pandolfi PP . Pten is essential for embryonic development and tumour suppression. Nat Genet 1998; 19: 348–355.
Wang S, Gao J, Lei Q, Rozengurt N, Pritchard C, Jiao J et al. Prostate-specific deletion of the murine Pten tumor suppressor gene leads to metastatic prostate cancer. Cancer Cell 2003; 4: 209–221.
Backman SA, Ghazarian D, So K, Sanchez O, Wagner KU, Hennighausen L et al. Early onset of neoplasia in the prostate and skin of mice with tissue-specific deletion of Pten. Proc Natl Acad Sci USA 2004; 101: 1725–1730.
Ma X, Ziel-van der Made AC, Autar B, van der Korput HA, Vermeij M, van Duijn P et al. Targeted biallelic inactivation of Pten in the mouse prostate leads to prostate cancer accompanied by increased epithelial cell proliferation but not by reduced apoptosis. Cancer Res 2005; 65: 5730–5739.
Trotman LC, Niki M, Dotan ZA, Koutcher JA, Di Cristofano A, Xiao A et al. Pten dose dictates cancer progression in the prostate. PLoS Biol 2003; 1: E59.
Ahmad I, Sansom OJ, Leung HY . Advances in mouse models of prostate cancer. Expert Rev Mol Med 2008; 10: e16.
Abate-Shen C, Banach-Petrosky WA, Sun X, Economides KD, Desai N, Gregg JP et al. Nkx3.1; Pten mutant mice develop invasive prostate adenocarcinoma and lymph node metastases. Cancer Res 2003; 63: 3886–3890.
Podsypanina K, Ellenson LH, Nemes A, Gu J, Tamura M, Yamada KM et al. Mutation of Pten/Mmac1 in mice causes neoplasia in multiple organ systems. Proc Natl Acad Sci USA 1999; 96: 1563–1568.
Bostwick DG, Brawer MK . Prostatic intra-epithelial neoplasia and early invasion in prostate cancer. Cancer 1987; 59: 788–794.
Bostwick DG . Prospective origins of prostate carcinoma. Prostatic intraepithelial neoplasia and atypical adenomatous hyperplasia. Cancer 1996; 78: 330–336.
Knobbe CB, Lapin V, Suzuki A, Mak TW . The roles of PTEN in development, physiology and tumorigenesis in mouse models: a tissue-by-tissue survey. Oncogene 2008; 27: 5398–5415.
Stambolic V, Suzuki A, de la Pompa JL, Brothers GM, Mirtsos C, Sasaki T et al. Negative regulation of PKB/Akt-dependent cell survival by the tumor suppressor PTEN. Cell 1998; 95: 29–39.
Sun H, Lesche R, Li DM, Liliental J, Zhang H, Gao J et al. PTEN modulates cell cycle progression and cell survival by regulating phosphatidylinositol 3,4,5,-trisphosphate and Akt/protein kinase B signaling pathway. Proc Natl Acad Sci USA 1999; 96: 6199–6204.
Coffer PJ, Jin J, Woodgett JR . Protein kinase B (c-Akt): a multifunctional mediator of phosphatidylinositol 3-kinase activation. Biochem J 1998; 335 (Pt 1): 1–13.
Datta SR, Brunet A, Greenberg ME . Cellular survival: a play in three Akts. Genes Dev 1999; 13: 2905–2927.
Kinkade CW, Castillo-Martin M, Puzio-Kuter A, Yan J, Foster TH, Gao H et al. Targeting AKT/mTOR and ERK MAPK signaling inhibits hormone-refractory prostate cancer in a preclinical mouse model. J Clin Invest 2008; 118: 3051–3064.
Chung JH, Eng C . Nuclear-cytoplasmic partitioning of phosphatase and tensin homologue deleted on chromosome 10 (PTEN) differentially regulates the cell cycle and apoptosis. Cancer Res 2005; 65: 8096–8100.
Koul HK, Maroni PD, Meacham RB, Crawford D, Koul S . p42/p44 Mitogen-activated protein kinase signal transduction pathway: a novel target for the treatment of hormone-resistant prostate cancer? Ann N Y Acad Sci 2004; 1030: 243–252.
Pascal LE, Ai J, Rigatti LH, Lipton AK, Xiao W, Gnarra JR et al. EAF2 loss enhances angiogenic effects of Von Hippel-Lindau heterozygosity on the murine liver and prostate. Angiogenesis 2011; 14: 331–343.
Folkman J . Tumor angiogenesis: therapeutic implications. N Engl J Med 1971; 285: 1182–1186.
Vivanco I, Palaskas N, Tran C, Finn SP, Getz G, Kennedy NJ et al. Identification of the JNK signaling pathway as a functional target of the tumor suppressor PTEN. Cancer cell 2007; 11: 555–569.
Maurus D, Heligon C, Burger-Schwarzler A, Brandli AW, Kuhl M . Noncanonical Wnt-4 signaling and EAF2 are required for eye development in Xenopus laevis. EMBO J 2005; 24: 1181–1191.
Liu JX, Hu B, Wang Y, Gui JF, Xiao W . Zebrafish eaf1 and eaf2/u19 mediate effective convergence and extension movements through the maintenance of wnt11 and wnt5 expression. J Biol Chem 2009; 284: 16679–16692.
Wan X, Ji W, Mei X, Zhou J, Liu JX, Fang C et al. Negative feedback regulation of Wnt4 signaling by EAF1 and EAF2/U19. PLOS One 2010; 5: e9118.
Mulholland DJ, Tran LM, Li Y, Cai H, Morim A, Wang S et al. Cell autonomous role of PTEN in regulating castration-resistant prostate cancer growth. Cancer Cell 2011; 19: 792–804.
Weidner N, Carroll PR, Flax J, Blumenfeld W, Folkman J . Tumor angiogenesis correlates with metastasis in invasive prostate carcinoma. Am J Pathol 1993; 143: 401–409.
Shappell SB, Thomas GV, Roberts RL, Herbert R, Ittmann MM, Rubin MA et al. Prostate pathology of genetically engineered mice: definitions and classification. The consensus report from the Bar Harbor meeting of the Mouse Models of Human Cancer Consortium Prostate Pathology Committee. Cancer Res 2004; 64: 2270–2305.
Weidner N . Current pathologic methods for measuring intratumoral microvessel density within breast carcinoma and other solid tumors. Breast Cancer Res Treat 1995; 36: 169–180.
Acknowledgements
This investigation was supported in part by the National Institutes of Health Grants R01 CA 120386, 5 R37 DK51193, 1 P50 CA90386 and T32 DK007774. This project used the UPCI Animal Facility and Tissue and Research Pathology Services (TARPS), and was supported in part by award P30CA047904. We thank Marie Acquafondata, Aiyuan Zhang, Katie Leschak and Dawn Everard (University of Pittsburgh, Pittsburgh, PA) for excellent technical assistance. We also thank all members of the Wang laboratory for insightful discussion and critical reading of the manuscript. LEP is a Tippins Foundation Scholar.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Ai, J., Pascal, L., O'Malley, K. et al. Concomitant loss of EAF2/U19 and Pten synergistically promotes prostate carcinogenesis in the mouse model. Oncogene 33, 2286–2294 (2014). https://doi.org/10.1038/onc.2013.190
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2013.190
Keywords
This article is cited by
-
EAF2 regulates DNA repair through Ku70/Ku80 in the prostate
Oncogene (2017)
-
A single-copy Sleeping Beauty transposon mutagenesis screen identifies new PTEN-cooperating tumor suppressor genes
Nature Genetics (2017)
-
Expression and prognostic significance of ELL-associated factor 2 in human prostate cancer
International Urology and Nephrology (2016)