Abstract
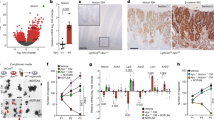
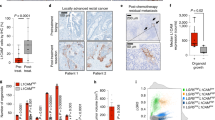
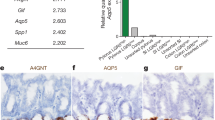
A gene signature specific for intestinal stem cells (ISCs) has recently been shown to predict relapse in colorectal cancer (CRC) but the tumorigenic role of individual signature genes remains poorly defined. A prominent ISC-signature gene is the cancer stem cell marker CD44, which encodes various splice variants comprising a diverse repertoire of adhesion and signaling molecules. Using Lgr5 as ISC marker, we have fluorescence-activated cell sorting-purified ISCs to define their CD44 repertoire. ISCs display a specific set of CD44 variant isoforms (CD44v), but remarkably lack the CD44 standard (CD44s) isoform. These CD44v also stand-out in transformed human ISCs isolated from microadenomas of familial adenomatous polyposis patients. By employing knock-in mice expressing either CD44v4-10 or CD44s, we demonstrate that the CD44v isoform, but not CD44s, promotes adenoma initiation in ApcMin/+mice. Our data identify CD44v as component of the ISCs program critical for tumor initiation, and as potential treatment target in CRC.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Noah TK, Donahue B, Shroyer NF . Intestinal development and differentiation. Exp Cell Res 2011; 317: 2702–2710.
Kuhnert F, Davis CR, Wang HT, Chu P, Lee M, Yuan J et al. Essential requirement for Wnt signaling in proliferation of adult small intestine and colon revealed by adenoviral expression of Dickkopf-1. Proc Natl Acad Sci USA 2004; 101: 266–271.
Nusse R . Wnt signaling and stem cell control. Cell Res 2008; 18: 523–527.
Kinzler KW, Vogelstein B . Lessons from hereditary colorectal cancer. Cell 1996; 87: 159–170.
Korinek V, Barker N, Morin PJ, van Wichen D, de Weger R, Kinzler KW et al. Constitutive transcriptional activation by a beta-catenin-Tcf complex in APC−/− colon carcinoma. Science 1997; 275: 1784–1787.
Morin PJ, Sparks AB, Korinek V, Barker N, Clevers H, Vogelstein B et al. Activation of beta-catenin-Tcf signaling in colon cancer by mutations in beta-catenin or APC. Science 1997; 275: 1787–1790.
Barker N, Ridgway RA, van Es JH, van de Wetering M, Begthel H, van den Born M et al. Crypt stem cells as the cells-of-origin of intestinal cancer. Nature 2009; 457: 608–611.
van de Wetering M, Sancho E, Verweij C, de Lau W, Oving I, Hurlstone A et al. The beta-catenin/TCF-4 complex imposes a crypt progenitor phenotype on colorectal cancer cells. Cell 2002; 111: 241–250.
Sansom OJ, Reed KR, Hayes AJ, Ireland H, Brinkmann H, Newton IP et al. Loss of Apc in vivo immediately perturbs Wnt signaling, differentiation, and migration. Genes Dev 2004; 18: 1385–1390.
van der Flier LG, Sabates-Bellver J, Oving I, Haegebarth A, De Palo M, Anti M et al. The intestinal Wnt/TCF signature. Gastroenterology 2007; 132: 628–632.
van der Flier LG, van Gijn ME, Hatzis P, Kujala P, Haegebarth A, Stange DE et al. Transcription factor achaete scute-like 2 controls intestinal stem cell fate. Cell 2009; 136: 903–912.
Merlos-Suarez A, Barriga FM, Jung P, Iglesias M, Cespedes MV, Rossell D et al. The intestinal stem cell signature identifies colorectal cancer stem cells and predicts disease relapse. Cell Stem Cell 2011; 8: 511–524.
Heider KH, Hofmann M, Hors E, van den Berg F, Ponta H, Herrlich P et al. A human homologue of the rat metastasis-associated variant of CD44 is expressed in colorectal carcinomas and adenomatous polyps. J Cell Biol 1993; 120: 227–233.
Wielenga VJ, Heider KH, Offerhaus GJ, Adolf GR, van den Berg FM, Ponta H et al. Expression of CD44 variant proteins in human colorectal cancer is related to tumor progression. Cancer Res 1993; 53: 4754–4756.
Mulder JW, Kruyt PM, Sewnath M, Oosting J, Seldenrijk CA, Weidema WF et al. Colorectal cancer prognosis and expression of exon-v6-containing CD44 proteins. Lancet 1994; 344: 1470–1472.
Zoller M . CD44: can a cancer-initiating cell profit from an abundantly expressed molecule? Nat Rev Cancer 2011; 11: 254–267.
Bennett KL, Modrell B, Greenfield B, Bartolazzi A, Stamenkovic I, Peach R et al. Regulation of CD44 binding to hyaluronan by glycosylation of variably spliced exons. J Cell Biol 1995; 131: 1623–1633.
van der Voort R, Taher TE, Wielenga VJ, Spaargaren M, Prevo R, Smit L et al. Heparan sulfate-modified CD44 promotes hepatocyte growth factor/scatter factor-induced signal transduction through the receptor tyrosine kinase c-Met. J Biol Chem 1999; 274: 6499–6506.
Jackson DG, Bell JI, Dickinson R, Timans J, Shields J, Whittle N . Proteoglycan forms of the lymphocyte homing receptor CD44 are alternatively spliced variants containing the v3 exon. J Cell Biol 1995; 128: 673–685.
Wielenga VJ, Smits R, Korinek V, Smit L, Kielman M, Fodde R et al. Expression of CD44 in Apc and Tcf mutant mice implies regulation by the WNT pathway. Am J Pathol 1999; 154: 515–523.
Dalerba P, Dylla SJ, Park IK, Liu R, Wang X, Cho RW et al. Phenotypic characterization of human colorectal cancer stem cells. Proc Natl Acad Sci USA 2007; 104: 10158–10163.
Zeilstra J, Joosten SP, Dokter M, Verwiel E, Spaargaren M, Pals ST . Deletion of the WNT target and cancer stem cell marker CD44 in Apc(Min/+) mice attenuates intestinal tumorigenesis. Cancer Res 2008; 68: 3655–3661.
Wielenga VJ, van der Neut R, Offerhaus GJ, Pals ST . CD44 glycoproteins in colorectal cancer: expression, function, and prognostic value. Adv Cancer Res 2000; 77: 169–187.
Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 2007; 449: 1003–1007.
van Weering DH, Baas PD, Bos JL . A PCR-based method for the analysis of human CD44 splice products. PCR Methods Appl 1993; 3: 100–106.
Boivin GP, Washington K, Yang K, Ward JM, Pretlow TP, Russell R et al. Pathology of mouse models of intestinal cancer: consensus report and recommendations. Gastroenterology 2003; 124: 762–777.
Orian-Rousseau V, Chen L, Sleeman JP, Herrlich P, Ponta H . CD44 is required for two consecutive steps in HGF/c-Met signaling. Genes Dev 2002; 16: 3074–3086.
Birchmeier C, Birchmeier W, Gherardi E, Vande Woude GF . Met, metastasis, motility and more. Nat Rev Mol Cell Biol 2003; 4: 915–925.
Di Renzo MF, Olivero M, Giacomini A, Porte H, Chastre E, Mirossay L et al. Overexpression and amplification of the met/HGF receptor gene during the progression of colorectal cancer. Clin Cancer Res 1995; 1: 147–154.
Wielenga VJ, van der Voort R, Taher TE, Smit L, Beuling EA, van Krimpen C et al. Expression of c-Met and heparan-sulfate proteoglycan forms of CD44 in colorectal cancer. Am J Pathol 2000; 157: 1563–1573.
Boon EM, van der Neut R, van de Wetering M, Clevers H, Pals ST . Wnt signaling regulates expression of the receptor tyrosine kinase met in colorectal cancer. Cancer Res 2002; 62: 5126–5128.
Vermeulen L, De Sousa EMF, van der Heijden M, Cameron K, de Jong JH, Borovski T et al. Wnt activity defines colon cancer stem cells and is regulated by the microenvironment. Nat Cell Biol 2010; 12: 468–476.
van der Voort R, Manten-Horst E, Smit L, Ostermann E, van den Berg F, Pals ST . Binding of cell-surface expressed CD44 to hyaluronate is dependent on splicing and cell type. Biochem Biophys Res Commun 1995; 214: 137–144.
Toole BP . Hyaluronan: from extracellular glue to pericellular cue. Nat Rev Cancer 2004; 4: 528–539.
Mantovani A, Allavena P, Sica A, Balkwill F . Cancer-related inflammation. Nature 2008; 454: 436–444.
Ramakers C, Ruijter JM, Deprez RH, Moorman AF . Assumption-free analysis of quantitative real-time polymerase chain reaction (PCR) data. Neurosci Lett 2003; 339: 62–66.
van der Neut R, Krimpenfort P, Calafat J, Niessen CM, Sonnenberg A . Epithelial detachment due to absence of hemidesmosomes in integrin beta 4 null mice. Nat Genet 1996; 13: 366–369.
Schmits R, Filmus J, Gerwin N, Senaldi G, Kiefer F, Kundig T et al. CD44 regulates hematopoietic progenitor distribution, granuloma formation, and tumorigenicity. Blood 1997; 90: 2217–2233.
McCart AE, Vickaryous NK, Silver A . Apc mice: models, modifiers and mutants. Pathol Res Pract 2008; 204: 479–490.
Acknowledgements
This work was supported by a grant from the Dutch Cancer Society. The authors declare no competing financial interests.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Zeilstra, J., Joosten, S., van Andel, H. et al. Stem cell CD44v isoforms promote intestinal cancer formation in Apc(min) mice downstream of Wnt signaling. Oncogene 33, 665–670 (2014). https://doi.org/10.1038/onc.2012.611
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2012.611
Keywords
This article is cited by
-
Intracellular pH dynamics regulates intestinal stem cell lineage specification
Nature Communications (2023)
-
Selection, characterization and in vivo evaluation of novel CD44v6-targeting antibodies for targeted molecular radiotherapy
Scientific Reports (2023)
-
Wnt signaling is boosted during intestinal regeneration by a CD44-positive feedback loop
Cell Death & Disease (2022)
-
Development of alternative splicing signature in lung squamous cell carcinoma
Medical Oncology (2021)
-
ILC1 drive intestinal epithelial and matrix remodelling
Nature Materials (2021)