Abstract
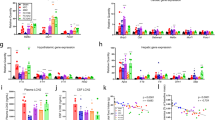
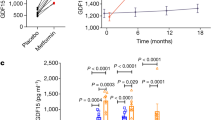
Leptin acts on its receptor (ObR) in the hypothalamus to inhibit food intake and energy expenditure. Leptin and ObR are also expressed in the gastrointestinal tract; however, the physiological significance of leptin signaling in the gut remains uncertain. Suppressor of cytokine signaling 3 (SOCS3) is a key negative feedback regulator of ObR-mediated signaling in the hypothalamus. We now show that gastrointestinal epithelial cell-specific SOCS3 conditional knockout (T3b-SOCS3 cKO) mice developed gastric tumors by enhancing leptin production and the ObRb/signal transducer and activator of transcription 3 (STAT3) signaling pathway. All T3b-SOCS3 cKO mice developed tumors in the stomach but not in the bowels by 2 months of age, even though the SOCS3 deletion occurred in both the epithelium of stomach and bowels. The tumors developed in the absence of the inflammatory response and all cKO mice died within 6 months. These tumors displayed pathology and molecular alterations, such as an increase in MUC2 (Mucin 2, oligomeric mucus/gel-forming) and TFF3 (trefoil factor 3), resembling human intestinal-type gastric tumors. Administration of antileptin antibody to T3b-SOCS3 cKO mice reduced hyperplasia of gastric mucosa, which is the step of the initiation of gastric tumor. These data suggest that SOCS3 is an antigastric tumor gene that suppresses leptin overexpression and ObRb/STAT3 hyperactivation, supporting the hypothesis that the leptin/ObRb/STAT3 axis accelerates tumorigenesis and that it may represent a new therapeutic target for the treatment of gastric cancer.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM . Positional cloning of the mouse obese gene and its human homologue. Nature 1994; 372: 425–432.
Friedman JM, Halaas JL . Leptin and the regulation of body weight in mammals. Nature 1998; 395: 763–770.
Bennett BD, Solar GP, Yuan JQ, Mathias J, Thomas GR, Matthews W . A role for leptin and its cognate receptor in hematopoiesis. Curr Biol 1996; 6: 1170–1180.
Park HY, Kwon HM, Lim HJ, Hong BK, Lee JY, Park BE et al. Potential role of leptin in angiogenesis: leptin induces endothelial cell proliferation and expression of matrix metalloproteinases in vivo and in vitro. Exp Mol Med 2001; 33: 95–102.
La Cava A, Matarese G . The weight of leptin in immunity. Nat Rev Immunol 2004; 4: 371–379.
Bado A, Levasseur S, Attoub S, Kermorgant S, Laigneau JP, Bortoluzzi MN et al. The stomach is a source of leptin. Nature 1998; 394: 790–793.
Sobhani I, Bado A, Vissuzaine C, Buyse M, Kermorgant S, Laigneau JP et al. Leptin secretion and leptin receptor in the human stomach. Gut 2000; 47: 178–183.
Morton NM, Emilsson V, Liu YL, Cawthorne MA . Leptin action in intestinal cells. J Biol Chem 1998; 273: 26194–26201.
Buyse M, Berlioz F, Guilmeau S, Tsocas A, Voisin T, Peranzi G et al. PepT1-mediated epithelial transport of dipeptides and cephalexin is enhanced by luminal leptin in the small intestine. J Clin Invest 2001; 108: 1483–1494.
Attoub S, Noe V, Pirola L, Bruyneel E, Chastre E, Mareel M et al. Leptin promotes invasiveness of kidney and colonic epithelial cells via phosphoinositide 3-kinase-, rho-, and rac-dependent signaling pathways. Faseb J 2000; 14: 2329–2338.
Tartaglia LA, Dembski M, Weng X, Deng N, Culpepper J, Devos R et al. Identification and expression cloning of a leptin receptor, OB-R. Cell 1995; 83: 1263–1271.
Bjorbaek C, Uotani S, da Silva B, Flier JS . Divergent signaling capacities of the long and short isoforms of the leptin receptor. J Biol Chem 1997; 272: 32686–32695.
Mix H, Widjaja A, Jandl O, Cornberg M, Kaul A, Goke M et al. Expression of leptin and leptin receptor isoforms in the human stomach. Gut 2000; 47: 481–486.
Ishikawa M, Kitayama J, Nagawa H . Expression pattern of leptin and leptin receptor (OB-R) in human gastric cancer. World J Gastroenterol 2006; 12: 5517–5522.
Bjorbak C, Lavery HJ, Bates SH, Olson RK, Davis SM, Flier JS et al. SOCS3 mediates feedback inhibition of the leptin receptor via Tyr985. J Biol Chem 2000; 275: 40649–40657.
Eyckerman S, Broekaert D, Verhee A, Vandekerckhove J, Tavernier J . Identification of the Y985 and Y1077 motifs as SOCS3 recruitment sites in the murine leptin receptor. FEBS Lett 2000; 486: 33–37.
Bjorbaek C, El-Haschimi K, Frantz JD, Flier JS . The role of SOCS-3 in leptin signaling and leptin resistance. J Biol Chem 1999; 274: 30059–30065.
Bjorbaek C, Elmquist JK, Frantz JD, Shoelson SE, Flier JS . Identification of SOCS-3 as a potential mediator of central leptin resistance. Mol Cell 1998; 1: 619–625.
Mori H, Hanada R, Hanada T, Aki D, Mashima R, Nishinakamura H et al. Socs3 deficiency in the brain elevates leptin sensitivity and confers resistance to diet-induced obesity. Nat Med 2004; 10: 739–743.
Zhao X, Huang K, Zhu Z, Chen S, Hu R . Correlation between expression of leptin and clinicopathological features and prognosis in patients with gastric cancer. J Gastroenterol Hepatol 2007; 22: 1317–1321.
Koda M, Sulkowska M, Kanczuga-Koda L, Surmacz E, Sulkowski S . Overexpression of the obesity hormone leptin in human colorectal cancer. J Clin Pathol 2007; 60: 902–906.
Feldman DE, Chen C, Punj V, Tsukamoto H, Machida K . Pluripotency factor-mediated expression of the leptin receptor (OB-R) links obesity to oncogenesis through tumor-initiating stem cells. Proc Natl Acad Sci USA 2012; 109: 829–834.
Sitaraman S, Liu X, Charrier L, Gu LH, Ziegler TR, Gewirtz A et al. Colonic leptin: source of a novel proinflammatory cytokine involved in IBD. FASEB J 2004; 18: 696–698.
Li Y, de Haar C, Chen M, Deuring J, Gerrits MM, Smits R et al. Disease-related expression of the IL6/STAT3/SOCS3 signalling pathway in ulcerative colitis and ulcerative colitis-related carcinogenesis. Gut 2010; 59: 227–235.
Rigby RJ, Simmons JG, Greenhalgh CJ, Alexander WS, Lund PK . Suppressor of cytokine signaling 3 (SOCS3) limits damage-induced crypt hyper-proliferation and inflammation-associated tumorigenesis in the colon. Oncogene 2007; 26: 4833–4841.
Banks AS, Davis SM, Bates SH, Myers MG . Activation of downstream signals by the long form of the leptin receptor. J Biol Chem 2000; 275: 14563–14572.
Thiery JP . Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer 2002; 2: 442–454.
Moody SE, Perez D, Pan TC, Sarkisian CJ, Portocarrero CP, Sterner CJ et al. The transcriptional repressor Snail promotes mammary tumor recurrence. Cancer Cell 2005; 8: 197–209.
Rosivatz E, Becker KF, Kremmer E, Schott C, Blechschmidt K, Hofler H et al. Expression and nuclear localization of Snail, an E-cadherin repressor, in adenocarcinomas of the upper gastrointestinal tract. Virchows Arch 2006; 448: 277–287.
Montero C, Segura DI . Retrospective histochemical study of mucosubstances in adenocarcinomas of the gastrointestinal tract. Histopathology 1980; 4: 281–291.
Lauren P . The two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma. An attempt at a histo-clinical classification. Acta Pathol Microbiol Scand 1965; 64: 31–49.
Suh E, Traber PG . An intestine-specific homeobox gene regulates proliferation and differentiation. Mol Cell Biol 1996; 16: 619–625.
Kim BM, Buchner G, Miletich I, Sharpe PT, Shivdasani RA . The stomach mesenchymal transcription factor Barx1 specifies gastric epithelial identity through inhibition of transient Wnt signaling. Dev Cell 2005; 8: 611–622.
Coussens LM, Werb Z . Inflammation and cancer. Nature 2002; 420: 860–867.
Du R, Lu KV, Petritsch C, Liu P, Ganss R, Passegue E et al. HIF1alpha induces the recruitment of bone marrow-derived vascular modulatory cells to regulate tumor angiogenesis and invasion. Cancer Cell 2008; 13: 206–220.
Wu L, Du H, Li Y, Qu P, Yan C . Signal transducer and activator of transcription 3 (Stat3C) promotes myeloid-derived suppressor cell expansion and immune suppression during lung tumorigenesis. Am J Pathol 2011; 179: 2131–2141.
He Y, Chen H, Quon MJ, Reitman M . The mouse obese gene. Genomic organization, promoter activity, and activation by CCAAT/enhancer-binding protein alpha. J Biol Chem 1995; 270: 28887–28891.
Zhang Y, Sif S, DeWille J . The mouse C/EBPdelta gene promoter is regulated by STAT3 and Sp1 transcriptional activators, chromatin remodeling and c-Myc repression. J Cell Biochem 2007; 102: 1256–1270.
Howard JM, Pidgeon GP, Reynolds JV . Leptin and gastro-intestinal malignancies. Obes Rev 2010; 11: 863–874.
Zhao L, Shen ZX, Luo HS, Shen L . Possible involvement of leptin and leptin receptor in developing gastric adenocarcinoma. World J Gastroenterol 2005; 11: 7666–7670.
Guilmeau S, Buyse M, Bado A . Gastric leptin: a new manager of gastrointestinal function. Curr Opin Pharmacol 2004; 4: 561–566.
Cinti S, Matteis RD, Pico C, Ceresi E, Obrador A, Maffeis C et al. Secretory granules of endocrine and chief cells of human stomach mucosa contain leptin. Int J Obes Relat Metab Disord 2000; 24: 789–793.
Stringer EJ, Pritchard CA, Beck F . Cdx2 initiates histodifferentiation of the midgut endoderm. FEBS Lett 2008; 582: 2555–2560.
Nduati V, Yan Y, Dalmasso G, Driss A, Sitaraman S, Merlin D . Leptin transcriptionally enhances peptide transporter (hPepT1) expression and activity via the cAMP-response element-binding protein and Cdx2 transcription factors. J Biol Chem 2007; 282: 1359–1373.
Ernst M, Najdovska M, Grail D, Lundgren-May T, Buchert M, Tye H et al. STAT3 and STAT1 mediate IL-11-dependent and inflammation-associated gastric tumorigenesis in gp130 receptor mutant mice. J Clin Invest 2008; 118: 1727–1738.
Judd LM, Bredin K, Kalantzis A, Jenkins BJ, Ernst M, Giraud AS . STAT3 activation regulates growth, inflammation, and vascularization in a mouse model of gastric tumorigenesis. Gastroenterology 2006; 131: 1073–1085.
Kidder BL, Yang J, Palmer S . Stat3 and c-Myc genome-wide promoter occupancy in embryonic stem cells. PLoS ONE 2008; 3: e3932.
Cascio S, Ferla R, D'Andrea A, Gerbino A, Bazan V, Surmacz E et al. Expression of angiogenic regulators, VEGF and leptin, is regulated by the EGF/PI3K/STAT3 pathway in colorectal cancer cells. J Cell Physiol 2009; 221: 189–194.
Aihara H, Hiwatashi N, Kumagai S, Obata Y, Shimosegawa T, Toyota T et al. The T3(b) gene promoter directs intestinal epithelial cell-specific expression in transgenic mice. FEBS Lett 1999; 463: 185–188.
Yasukawa H, Ohishi M, Mori H, Murakami M, Chinen T, Aki D et al. IL-6 induces an anti-inflammatory response in the absence of SOCS3 in macrophages. Nat Immunol 2003; 4: 551–556.
Inagaki-Ohara K, Sasaki A, Matsuzaki G, Ikeda T, Hotokezaka M, Chijiiwa K et al. Suppressor of cytokine signalling 1 in lymphocytes regulates the development of intestinal inflammation in mice. Gut 2006; 55: 212–219.
Inagaki-Ohara K, Sakamoto Y, Dohi T, Smith AL . γδ T cells play a protective role during infection with Nippostrongylus brasiliensis by promoting goblet cell function in the small intestine. Immunology 2011; 134: 448–458.
Inagaki-Ohara K, Chinen T, Matsuzaki G, Sasaki A, Sakamoto Y, Hiromatsu K et al. Mucosal T cells bearing TCR γδ play a protective role in intestinal inflammation. J Immunol 2004; 173: 1390–1398.
Acknowledgements
This work was supported by grants from Grants-in-Aid for Scientific Research (C) from the Ministry of Education, Culture, Sports, Science and Technology of Japan (17590373, 19590432, 20599015 and 23590272), the Takeda Science Foundation and the Public Trust Haraguchi Memorial Cancer Research Fund (to KI-O) and Grants-in-aid for Scientific Research (S) from the Ministry of Education, Science, Technology, Sports, and Culture of Japan (to AY). We greatly thank Dr Jun-ichi Miyazaki (Osaka University) for the gift of the T3b-IL-12 vector, Ms Kozue Takahashi and Mr Takashi Kanna (University of the Ryukyus) for animal care, Dr Atsuo T Sasaki (Harvard University) for help in creating the T3b-cre construction, Dr Richard Blumberg (Harvard Medical School) and Dr Mayu Inaba (University of Michigan) for their constructive discussion and Dr Adrian L Smith (Oxford University) for reading carefully. We also thank Dr Takeshi Arakawa (University of the Ryukyus), Dr Shiki Okamoto, Ms Kumiko Saito (NIPS), Dr Miwa Tamura-Nakano and Dr Takeshi Nitta (NCGM) for steadfast supporting and encouraging us.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Oncogene website
Rights and permissions
About this article
Cite this article
Inagaki-Ohara, K., Mayuzumi, H., Kato, S. et al. Enhancement of leptin receptor signaling by SOCS3 deficiency induces development of gastric tumors in mice. Oncogene 33, 74–84 (2014). https://doi.org/10.1038/onc.2012.540
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2012.540
Keywords
This article is cited by
-
E3 ligase SOCS3 regulates NOD2 expression by ubiquitin proteasome system in lung cancer progression
Cellular Oncology (2023)
-
Leptin receptor signaling is required for high-fat diet-induced atrophic gastritis in mice
Nutrition & Metabolism (2016)
-
SOCS3 revisited: a broad regulator of disease, now ready for therapeutic use?
Cellular and Molecular Life Sciences (2016)
-
Lymph node metastasis is mediated by suppressor of cytokine signaling-3 in gastric cancer
Tumor Biology (2013)